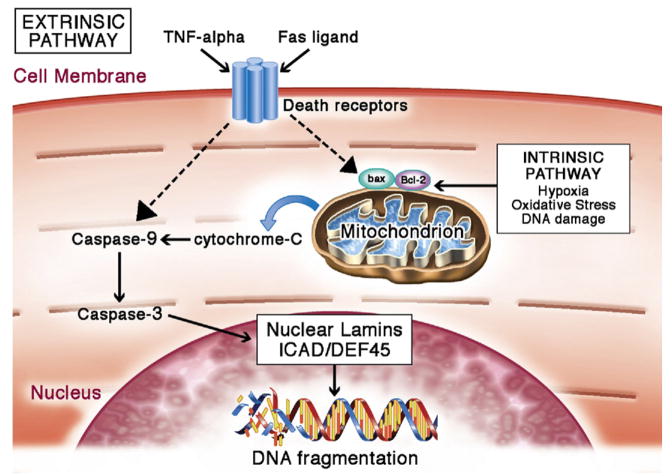
Figure 4. Cardiomyocyte Apoptosis.
Apoptosis can be mediated by the intrinsic or extrinsic pathways. Many factors associated with these pathways are increased with aging, resulting in a predisposition toward apoptosis in the aging cardiomyocyte. Intrinsically, apoptosis is activated in the event of hypoxia, oxidative stress, DNA damage, acidosis, or growth factor deprivation (36,37). These stressors activate cell death signaling chiefly through Bcl-2 family–regulated increase of mitochondrial outer membrane permeabilization (MOMP). Cytochrome C from mitochondria diffuses into the cytoplasm following the increase in mitochondrial membrane permeability. In the cytosol, cytochrome C activates a downstream cascade to initiate caspase activities (38,39). Active caspases target nuclear lamins, nuclear inhibitor of caspase-activated deoxyribonuclease/DNA fragmentation factor-45 (ICAD/DEF45), and various other cytoplasmic proteins. Cleavage of nuclear lamins breaks down the nuclear structure (40). Cleavage of ICAD/DEF45, a DNase inhibitor, disinhibits DNase, thereby allowing it to break down DNA (41). Extrinsic apoptosis can be activated by Fas ligand or tumor necrosis factor (TNF)-alpha. Binding of TNF-alpha or Fas ligand to their respective receptors activates TNF receptor–associated death domain (TRADD) and Fas-associated death domain (FADD) proteins (42,43). These proteins act on regulators of mitochondria to change the permeability of the mitochondrial outer membrane, and this change converges with intrinsic apoptosis activation on the caspase cascade. Figure illustration by Craig Skaggs.