Figure 1. MMP-2 and -9 activities co-purified with FN from human plasma by gelatin-Sepharose affinity chromatography.
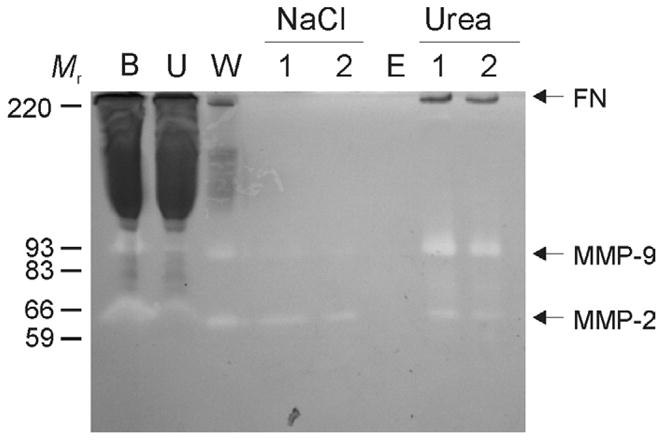
Residual gelatinolytic activities, including MMP-2 and -9, were monitored by gelatin substrate enzymography in column fractions during purification of fibronectin (FN). For analytical purification 500 μl of human plasma (B) was loaded on a gelatin Sepharose (Vt 25 μ1) mini column after sedimentation of insoluble aggregates at 2,500 × g. After collection of unbound proteins (U), the column was thoroughly washed (20 × Vt) with chromatography buffer (W; 50 mM Tris, 0.15 M NaCl, pH7.4) followed by 1M NaCl (N; 1, 2) in chromatography buffer (10 × Vt) to remove non-specifically bound proteins. After equilibration (E), specifically bound proteins were eluted with 4M urea (U; 1, 2). The enzymography analysis showed that plasma-derived MMP-2 and -9 (B) bound gelatin Sepharose and were co-purified with FN. Positions of FN, MMP-2, MMP-9, and kDa of proteins (Mr) are indicated.
