Figure 3. Heat treatment enhances FN cleavage by MMP-2 and blocks autolysis of FN.
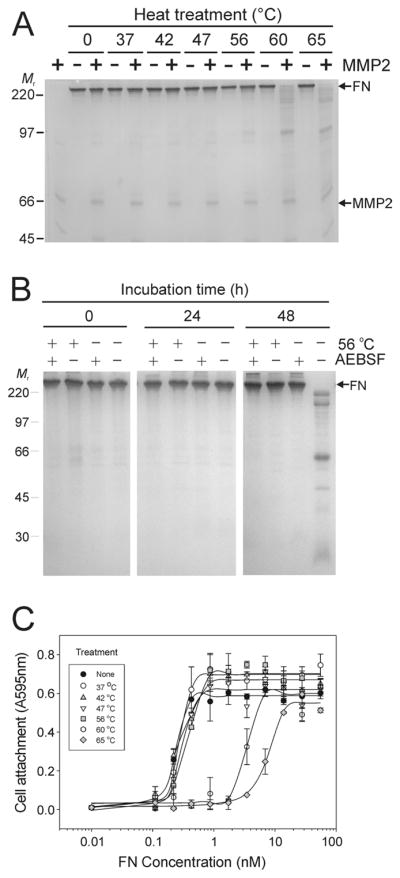
Panel A. Experiments tested the effects of heat treatment on the stability and susceptibility of FN to MMP-2 cleavage. Preparations of FN without detectable plasma MMP-2 or -9 were subjected to limited (30 min) heat treatment between 37 and 65 °C, and then incubated in the presence or absence of exogenous recombinant MMP-2 for 24 h at 22 °C. Analysis by SDS-PAGE showed substantial cleavage of FN samples treated at 56 °C with increasing extent of hydrolysis after treatment at 60 and 65 °C. Panel B. Control FN samples or samples treated for 30 min at 56 °C were subsequently incubated at 22 °C in the presence or absence of AEBSF. Analysis by SDS-PAGE detected only autolysis after 48 h in FN samples that were not heat-treated in the absence of AEBSF, whereas samples containing AEBSF were stable. There were no signs of autolysis of FN after heat-treatment irrespective of the presence or absence of AEBSF implying that heat-treatment inactivated inherent autolytic activities. Panel C. The functional integrity of heat-treated FN was analyzed in cell attachment assays. HT 1080 fibrosarcoma cells in serum-free medium were seeded in 96 microwell tissues culture plates coated with a concentration range of FN samples (70 - 0.01 nM) treated between 37 and 65 °C or control FN. Cell attachment was quantified at 595 nm from re-dissolved crystal violet dye after staining of cells. Cell attachment was comparable to control for FN samples heated up to 56 °C, but substantially reduced when FN was treated at 60 or 65 °C. Positions of FN (FN) and recombinant MMP-2 (MMP2), and kDa of protein standards (Mr) are presented.
