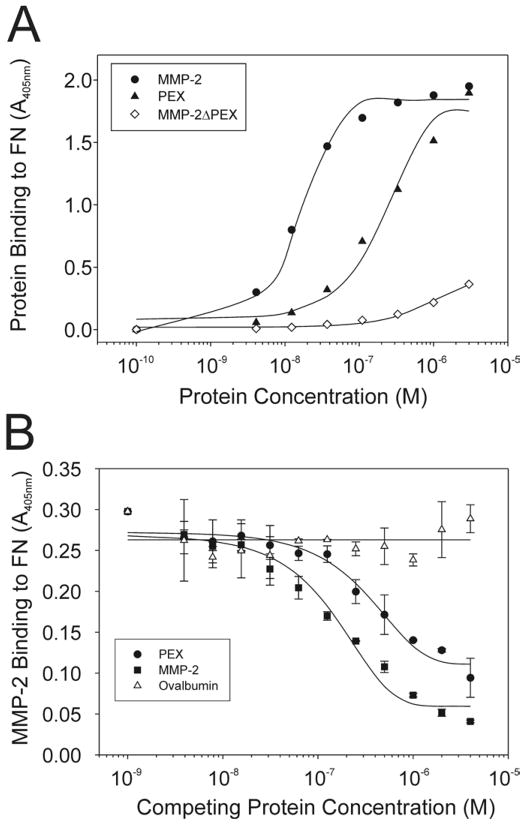
Figure 4. Interaction of FN with the carboxyl-terminal domain of MMP-2.
Panel A. Interactions of FN with the carboxyl-terminal hemopexin-like domain of MMP-2 (PEX) were analyzed. FN was coated in 96 microwell plates (0.5 μg/well), blocked with BSA (10 mg/ml), and reacted with a concentration range (3 × 10−6 – 10−10 M) of biotinylated MMP-2, PEX, or MMP-2ΔPEX in which the PEX is deleted. Protein binding to coated FN was detected with alkaline phosphatase-conjugated streptavidin and PNPP substrate, and quantified at 405 nm. MMP-2 and PEX bound in a concentration-dependent saturable manner to FN. In comparison, MMP-2ΔPEX showed weak and non-saturable binding to this ligand. Panel B. The specific contribution of PEX to the binding was measured in competitive protein-protein binding assays in which biotinylated MMP-2 was added simultaneously with a concentration range of unlabeled MMP-2, PEX or ovalbumin. Like unlabeled MMP-2 (positive control), isolated PEX competitively inhibited the binding of MMP-2 to FN in a concentration-dependent manner pointing to PEX as an essential exosite for MMP-2 interactions with FN.