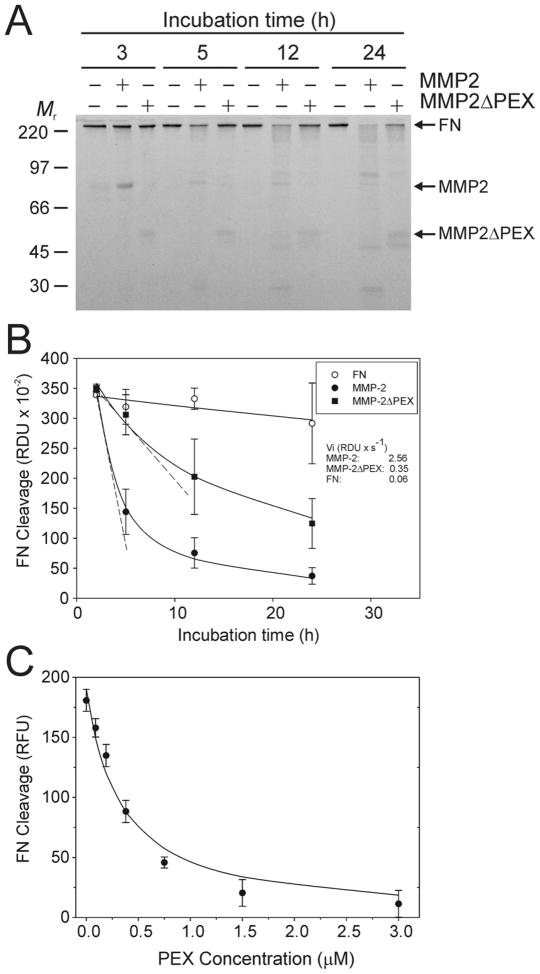
Figure 5. Contribution of the carboxyl-terminal domain to cleavage of FN by MMP-2.
The role of the carboxyl-terminal domain (PEX) of MMP-2 to cleavage of FN by the enzyme was evaluated using recombinant wildtype MMP-2, MMP-2ΔPEX which lacks the PEX, and isolated PEX in regular and competitive enzyme activity assays. Panel A. FN (100 μg/ml) after heat-treatment at 56 °C for 30 min were incubated with MMP-2 (MMP-2, +) or MMP-2ΔPEX (MMP-2ΔPEX, +) for 3 - 24 h at 22 °C. Panel B. Equal aliquots of the reactions collected at designated time points were separated by SDS-PAGE. FN cleavage was quantified by densitometric analysis of the intact FN on the gels and plotted for each enzyme as well as control by relative densitometric units (RDU) as a function of time. The rate of FN cleavage (RDU × s−1) determined in the linear range of the assays is presented. Panel C. Assays analyzed the capacity of a concentration range (0 and 0.05 – 3.0 μM) exogenous PEX to inhibit degradation of FITC-labeled FN by MMP-2. Relative fluorescent units (RFU) representing the extent of FN cleavage recorded in the linear range of the assays after 30 min at 22 °C are presented. PEX inhibited FN cleavage by MMP-2 in a concentration dependent manner. Together, these results demonstrated that PEX contributes essential exodomain functions to MMP-2 cleavage of FN. The positions of FN, MMP-2, MMP-2ΔPEX, and kDa of proteins (Mr) are shown.