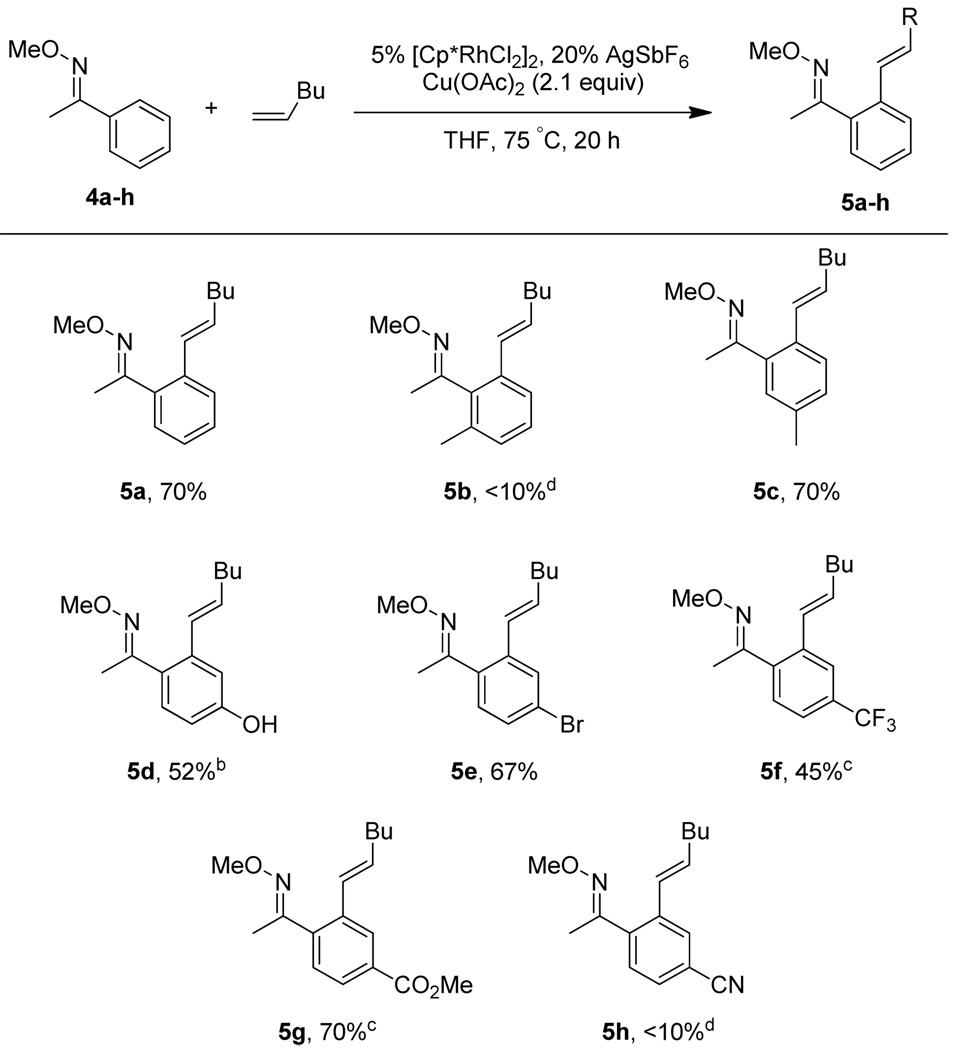
Scheme 1.
Aryl O-Methyl Oxime Scopea
a All reactions were performed by heating oxime (1 equiv), 1-hexene (3 equiv), [Cp*RhCl2]2 (5 mol %), AgSbF6 (20 mol %), Cu(OAc)2 (2.1 equiv), and THF (0.1 M) in a sealed vial for 20 h at 75 °C. Isolated yields after purification by chromatography are reported. b 1-hexene (1.5 equiv). c 36 h. d NMR yield relative to 2,6-dimethoxytoluene as an internal standard