Figure 7.
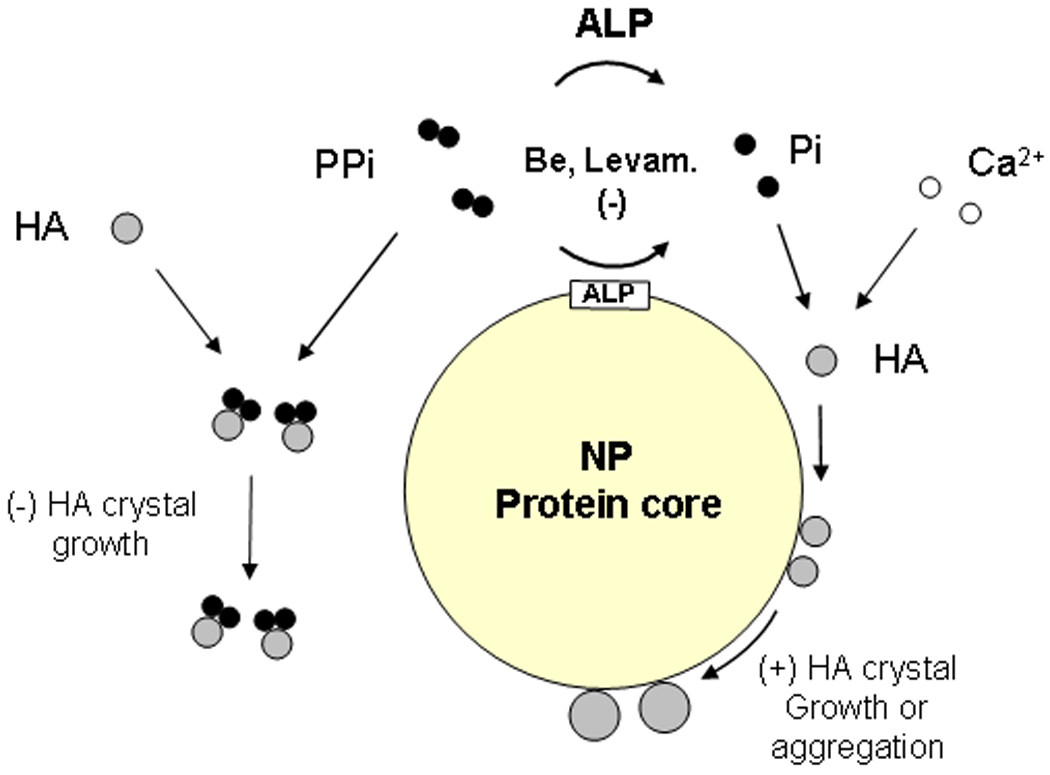
Possible relationships among proteins involved with formation and mineralization of NPs. Fetuin-A and albumin of likely bovine origin and other proteins with known affinity for calcium phosphate crystals have been identified in the protein core of NPs. Proteins of prokaryotic origin, considered to be important factors in metabolism, such as EF-Tu and EF-G, also appear to be present. ALP, which is associated with NPs and in plasma/extracellular space, hydrolyzes PPi and other substrates, including nucleotide triphosphates. The hydrolysis is inhibited by beryllium and levamisole. The liberated Pi, together with Ca2+ forms small HA crystals. PPi binds to some of the crystals, inhibiting their growth, whereas other crystals bind to the NP protein(s). Crystals bound to certain NP proteins may continue to grow by increasing mineral deposition. Alternatively, NPs may aggregate, forming larger protein/mineral complexes.
