Table 2.
CDR of rac-1 and Negishi arylation or vinylation
 | ||||
|---|---|---|---|---|
| entry | electrophile | product | yield (%) |
er (R:S) |
| 1a | 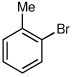 |
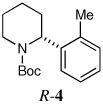 |
63 | 92:8 |
| 2a,b | 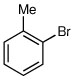 |
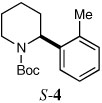 |
59 | 6:94 |
| 3a | 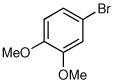 |
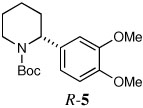 |
75 | 97:3 |
| 4a | 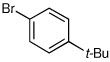 |
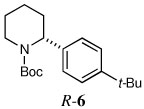 |
69 | 95:5 |
| 5a | 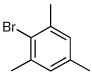 |
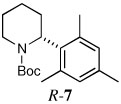 |
64 | 92:8 |
| 6a | 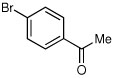 |
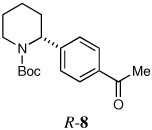 |
60 | 91:9 |
| 7a,c | 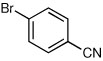 |
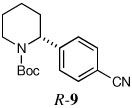 |
66 | 90:10 |
| 8a | 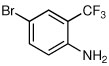 |
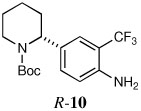 |
60 | 93:7 |
| 9e | 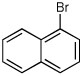 |
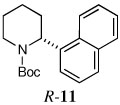 |
67 | 97:3 |
| 10d | 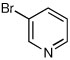 |
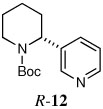 |
46 | 88:12 |
| 11b,d | 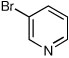 |
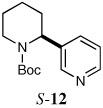 |
51 | 10:90 |
| 12d | 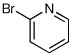 |
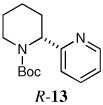 |
50 | 93:7 |
| 13d | 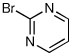 |
 |
53 | 85:15 |
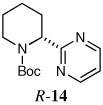
| 14a | 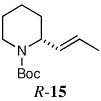 |
63 | 92:8 | |
| 15a,e | 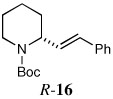 |
66 | 93:7 | |
stirred at rt for 18 h during step (iv),
CDR using (S,R)-2;
the aryl bromide contained a small amount of 2-bromobenzonitrile leading to formation of the ortho cyano constitutional isomer in 90:10 er;
stirred at 60 °C for 22 h during step (iv),
a small amount of the Z-alkene was obtained as a minor isomer in 92:8 er.
