Table 1.
Dissociation constants for OHPCBs with rSULT1A1 that had been pretreated with either GSH or GSSG
| OHPCBs | Chemical structure |
IC50a (µM) |
Kd values for OHPCBs with rSULT1A1 (µM)b | |||
|---|---|---|---|---|---|---|
| Without PAP | With 0.27 mM PAP | |||||
| GSH | GSSG | GSH | GSSG | |||
| 4’-OH PCB 9 | 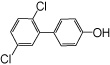 |
—c | 12 ± 4 | 7.1 ± 1.3 | 3.1 ± 1.5 | 3.3 ± 0.1 |
| 4’-OH PCB 12 | —c | 4.0 ± 0.8e | 2.5 ± 0.7e | 3.1 ± 0.01 | 4.6 ± 1.2e | |
| 6’-OH PCB 35 |
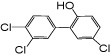
|
—c | 65 ± 32 | 117 ± 36 | 3.7 ± 0.01e | 3.6 ± 1.3e |
| 4’-OH PCB 35 |
|
— c | 3.6 ± 0.9 | 1.6 ± 0.4 | 1.6 ± 0.1 | 1.7 ± 0.1 |
| 4’-OH PCB 6 |
|
23d | 0.66 ± 0.2 | 0.50 ± 0.1 | 0.88 ± 0.03 | 0.72 ± 0.01 |
| 4’-OH PCB 33 |
|
26 d | 2.0 ± 0.1 | 2.5 ± 0.4 | 2.0 ± 0.01 | 2.4 ± 0.04 |
| 4-OH PCB 14 |
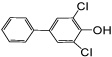
|
0.27 d | 0.26 ± 0.03 | 0.22 ± 0.02 | 0.40 ± 0.05f | 0.29 ± 0.04f |
| 4’-OH PCB 68 |
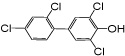
|
0.85 | 0.67 ± 0.1 | 0.53 ± 0.1 | 0.47 ± 0.02 | 0.40 ± 0.04 |
| 4-OH PCB 34 |
|
0.34 | 0.37 ± 0.01 | 0.23 ± 0.01e | 0.49g | 0.54 ± 0.01 |
IC50 values with rSULT1A1 (all cysteines in the thiol form) were determined previously with 2-naphthol as the substrate [7].
Kd values were determined utilizing rSULT1A1 that had been pretreated with either 1 mM GSH or 1 mM GSSG as described in section 2.4, and, unless otherwise indicated, data were fit to a one-site saturation equation with correction for non-specific binding of the ANS probe. Values are means and S.E. of two determinations unless otherwise indicated.
This OHPCB is a substrate for rSULT1A1.
This OHPCB is an inhibitor for the enzyme pretreated with GSH, but it is a substrate for rSULT1A1 pretreated with 1 mM GSSG.
The Kd value shown is the first Kd when the data were fit to a two-site equation; the second Kd was over 35 times greater than the first Kd.
Three determinations were performed.
A single determination was performed.
