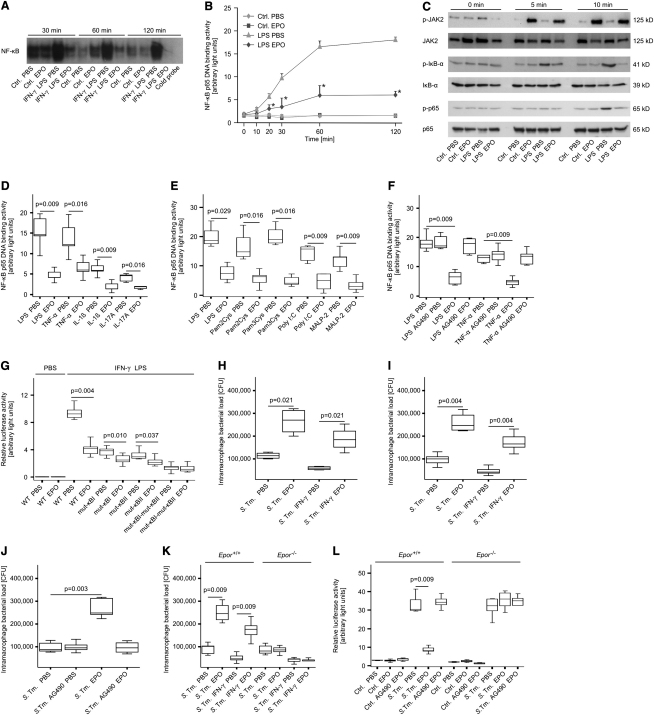
Figure 2.
Recombinant EPO Impairs NF-κB Activation and Salmonella Elimination In Vitro
(A) RAW264.7 macrophage-like cells were treated with EPO, IFN-γ, and LPS for the indicated time periods. NF-κB DNA binding activity in nuclear extracts was evaluated by means of electrophoretic mobility shift assay (EMSA). The specificity of binding was confirmed by cold competition with a 30-fold excess of the same unlabeled oligonucleotide (far right lane) with the nuclear extract of PBS-treated cells stimulated with IFN-γ and LPS for 120 min. One of three representative experiments is shown.
(B) Nuclear proteins were used for specific quantification of NF-κB p65 binding activity by a commercially available chemi-luminescent transcription factor assay at the indicated time points. Data of three independent experiments are expressed as arbitrary light units and shown as means ± SEM and were compared by means of Kruskal-Wallis test. Asterisks indicate statistically significant differences between LPS-stimulated cells pretreated with either PBS or EPO: p < 0.05.
(C) Cytoplasmic proteins from parallel experiments described in the legend for Figure 2B were used for evaluating phosphorylation of JAK2, IκB-α, and of NF-κB p65 by means of western blot as described in Experimental Procedures. Total JAK2, IκB-α, and NF-κB p65 protein, respectively, served as loading controls. One of three representative experiments is shown.
(D–F) RAW 264.7 cells were pretreated with PBS or EPO as above and subsequently stimulated with LPS or recombinant murine cytokines (D). Alternatively, RAW cells were treated with EPO and the Toll-like receptor (TLR) ligands LPS, PAM2Cys, PAM3Cys, Poly(I:C), or MALP-2 (E). RAW cells were treated with LPS or TNF-α after preincubation with the specific JAK2 inhibitor AG490 (F). Nuclear proteins were used for specific quantification of NF-κB p65 binding activity by a commercially available chemi-luminescent transcription factor assay after 120 min. Data of five independent experiments are depicted as arbitrary light units and shown as means ± SEM.
(G) RAW264.7 cells were transiently transfected with murine Nos2 promoter constructs carrying site-specific mutations in one or both NF-κB binding sites (mut-κBI-Nos2-luc and mut-κBII-Nos2-luc or mut-κBI-mut-κBII-Nos2-luc, respectively). Thereafter, macrophages were stimulated with IFN-γ and LPS after preincubation with EPO or solvent. Luciferase activity was measured in a chemi-luminometer and is shown as arbitrary light units.
(H–K) RAW264.7 macrophage-like cells (H) or primary peritoneal macrophages (I-K) were infected with S. typhimurium (S. Tm.) at a MOI of 10. After 1 hr, cells were treated with EPO, IFN-γ, AG490 or solvent and incubated for a total of 24 hr. Thereafter, macrophages were lysed and intramacrophage bacteria were enumerated by plating serial dilutions of cell lysates.
(L) Primary peritoneal macrophages from Epor+/+ and Epor−/− mice were treated as above after transient transfection with a NF-κB reporter construct. Data of five independent experiments are depicted.