Abstract
Transgenic and knockout mouse models have proven useful in the study of genes necessary for parturition—including genes that affect the timing and/or progression of labor contractions. However, taking full advantage of these models will require a detailed characterization of the contractile patterns in the mouse uterus. Currently the best methodology for this has been measurement of isometric tension in isolated muscle strips in vitro. However, this methodology does not provide a real-time measure of changes in uterine pressure over the course of pregnancy. Recent advances have opened the possibility of using radiotelemetric devices to more accurately and comprehensively study intrauterine pressure in vivo. We tested the effectiveness of this technology in the mouse, in both wild-type (WT) mice and a mouse model of defective parturition (SK3 channel-overexpressing mice), after surgical implant of telemetry transmitters into the uterine horn. Continuous recordings from day 18 of pregnancy through delivery revealed that WT mice typically deliver during the 12-h dark cycle after 19.5 days postcoitum. In these mice, intrauterine pressure gradually increases during this cycle, to threefold greater than that measured during the 12-h cycle before delivery. SK3-overexpressing mice, by contrast, exhibited lower intrauterine pressure over the same period. These results are consistent with the outcome of previous in vitro studies, and they indicate that telemetry is an accurate method for measuring uterine contraction, and hence parturition, in mice. The use of this technology will lead to important novel insights into changes in intrauterine pressure during the course of pregnancy.
Keywords: potassium channel, SK3 channel-overexpressing mice, preterm labor, uterine contraction, myometrium
preterm deliveries alone account for over 12.6% of all births in the U.S. and are associated with a high percentage (∼85%) of perinatal morbidity and mortality (2). Despite increased medical intervention over the past 30 years, preterm birth rates have risen by 28%—with most of these births occurring in women who do not have any of the known risk factors. Risk factors that have been identified are related to four distinct processes, each of which activates multiple signaling pathways and leads to preterm birth: precocious fetal endocrine activation, uterine overdistension, decidual bleeding, and intrauterine inflammation/infection (14).
Genetically modified mouse models have served as effective systems in which to investigate the signaling pathways essential to labor—both preterm and term. Despite certain differences in parturition between human and mouse, these species are similar with respect to many of the pathways that are known to modify pregnancy outcome (8). Mouse models of altered parturition include those in which myometrial contraction, progesterone withdrawal, abnormal cervical ripening, or the circadian rhythm is affected (12). While alterations in individual genes can affect different pathways to parturition, all processes must ultimately lead to a change in myometrial contraction. The characterization of changes in uterine contraction that are associated with abnormal pregnancy in the mouse has thus far been limited to the measurement of isometric tension. Although these measurements can provide important information, they are restricted to one stage of pregnancy rather than giving a comprehensive view of contractile and pressure changes over the course of pregnancy and delivery. Furthermore, this assay requires that the animals are euthanized, and thus precludes an assessment of the innate progression of labor or its initiation. These technical limitations have hindered the amount of information that can be obtained from these mouse models.
Recently, studies in rats demonstrated that radiotelemetry could be used to measure intrauterine pressures in vivo (6, 13). Although the size of the radiotelemetric transmitters initially excluded use of this technology to measure intrauterine pressure in mice, the development of a miniature transmitter (1.1 cc) that can measure physiological pressure (in the range of −20 to 300 mmHg) has enabled us to develop a method that can be utilized to measure intrauterine pressure in mice. We have now applied this procedure to SK3 channel-overexpressing (SK3T/T) mice, in which uterine contractions are dampened, resulting in delayed or hindered delivery in 70% of dams (1, 10). In addition, we further distinguished the role that SK3 channels play in the labor process. These studies demonstrate that radiotelemetry can be used effectively to study uterine contractions, as part of the full characterization of genetic mouse models of disrupted labor.
METHODS
Animals.
All animal procedures complied with the guidelines for the care and use of animals set forth by the National Institutes of Health. The Animal Care and Use Committee at the University of Iowa approved all protocols. SK3T/T mice (gift from John Adelman at the Vollum Institute) were on a C57BL/6J background. Wild-type (WT) littermates of SK3T/T mice served as controls. Adult female mice were mated at 8 wk of age or later. Mice were mated for 2-h time periods, and the presence of a copulatory plug was marked 0 days postcoitum (dpc). Animals were housed in Soft-Zorb bedding (Nepco, Warrensburg, NY) and fed standard mouse chow (Harlan Laboratories, 7913).
Surgical procedure.
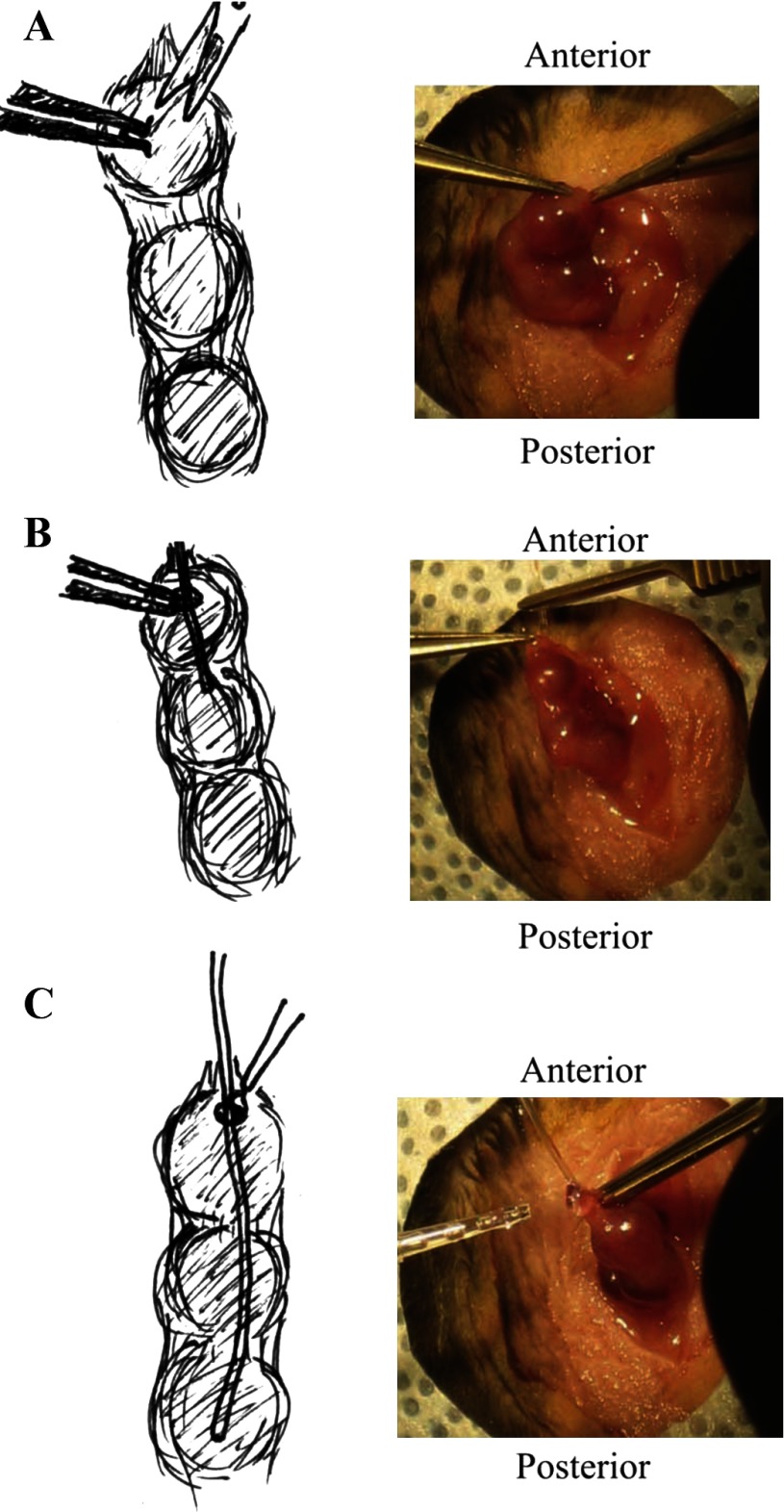
Originally designed for measuring blood pressure in mice (7), the PhysioTel PA-C10 transmitter [Data Sciences International (DSI), St. Paul, MN] was used to measure intrauterine pressure during labor. Before implantation, PA-C10 transmitters were cleaned and sterilized according to DSI instructions. Mice were weighed and injected with buprenorphine (3 μg) before surgery. Animals were anesthetized with a ketamine (91 mg/kg) and xylazine (9.1 mg/kg ip) mix. With aseptic and sterile technique, an incision ∼1 in. long was made in the abdomen wall (Fig. 1A). The uterus was carefully exteriorized, and the number of pups in each uterine horn was counted. One horn was selected for catheter implantation, and a small incision was made at the top of the uterine horn (Fig. 1A). Vessel cannulation forceps (World Precision Instruments, Sarasota, FL) were used to carefully thread the pressure catheter into the uterine horn, between the uterine wall and fetus (Fig. 1B). A small drop of Vetbond (3M, St. Paul, MN) was applied to the site of insertion and allowed to dry (Fig. 1C). The transmitter was carefully placed into the lower portion of the abdominal cavity to avoid damage to the liver, and the implanted horn was reinserted into the body cavity. The abdominal wall was then sutured closed, and the mouse was monitored until fully awake. Soft food was available to animals from after surgery through to postdelivery to improve maternal outcome.
Fig. 1.
Surgical procedure for implanting uterine telemetry transmitters. A: a small cut is made at top of the uterine horn. B: the pressure-catheter lead is carefully pushed into the uterine horn between the fetal sac and uterine wall. C: a small drop of Vetbond is applied to the incision site to secure the catheter. The transmitter body is placed inside the lower portion of the body cavity, and the uterine horn is replaced into the body cavity without disturbing the transmitter implant.
Induction of preterm labor in WT mice.
To test whether uterine telemetry can be used successfully to monitor labor in an induced model of preterm labor, we implanted WT mice as described above. Recordings were started on 14 dpc, and at 1:00 PM on 15 dpc the mice were injected with RU486 (100 μg; Sigma, St. Louis, MO) in ethanol, as described previously (4, 10). Uterine pressure was measured for 24 h.
Data acquisition and statistical analysis.
Telemetry recordings were performed at 500 Hz with Dataquest ART data acquisition system version 4.10 (DSI). Continuous recordings were begun on 18 dpc (14 dpc in preterm labor model) and continued through 22 dpc or until after delivery, whichever occurred first. The light and dark cycles of the mice were noted for each delivery. The changes in pressure between the 12-h cycle in which the mouse delivered were compared with those during the 12-h cycle preceding delivery. Hourly averages were calculated with Dataquest A.R.T. 4.2 software (DSI), which averaged all data points acquired within an hour.
Data gathered by the telemetry transmitters during the 12-h light or dark cycle preceding delivery and the 12-h light or dark cycle in which delivery occurred were used to examine the power spectrum of the pressure as fast Fourier transformations generated with Dataquest A.R.T. 4.2 software (DSI). This allows us to capture the frequency information of the pressure oscillations and identify periodicities. All out-of-range data points (points the software was unable to record as a numerical value) were replaced by points that were calculated from interpolation between adjacent points. Results from the fast Fourier transformation analysis ranged between 0.00039 and 0.03398 Hz (i.e., events with periods ranging from 29.4 s to 42 min, 44 s). The power spectrum of pressures calculated from the 12-h cycle in which delivery occurred was normalized for each frequency bin by the power calculated from the 12-h cycle before delivery for each animal.
All data are presented as means ± SE. In all cases, n refers to the number of animals. Statistical significance was determined by one-way ANOVA, or two-way ANOVA where appropriate, followed by post hoc comparison using Student's t-tests. Significance was determined at P < 0.05.
RESULTS
Intrauterine pressure increases during labor in WT mice.
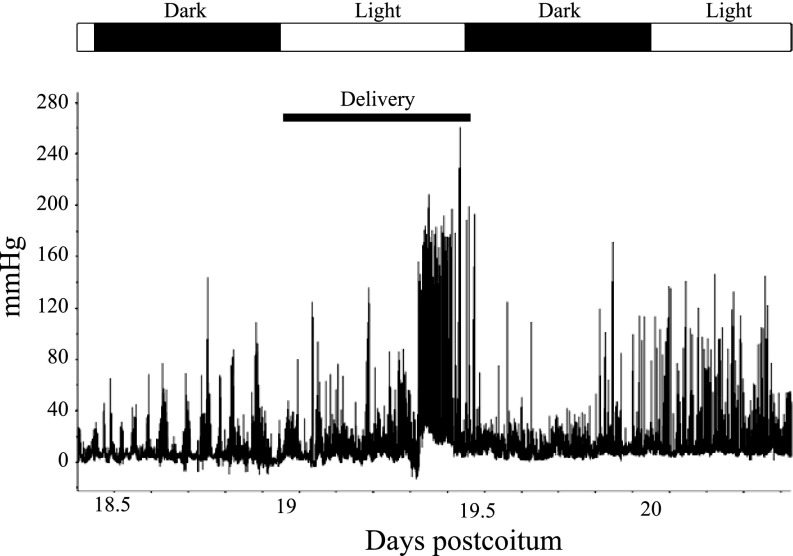
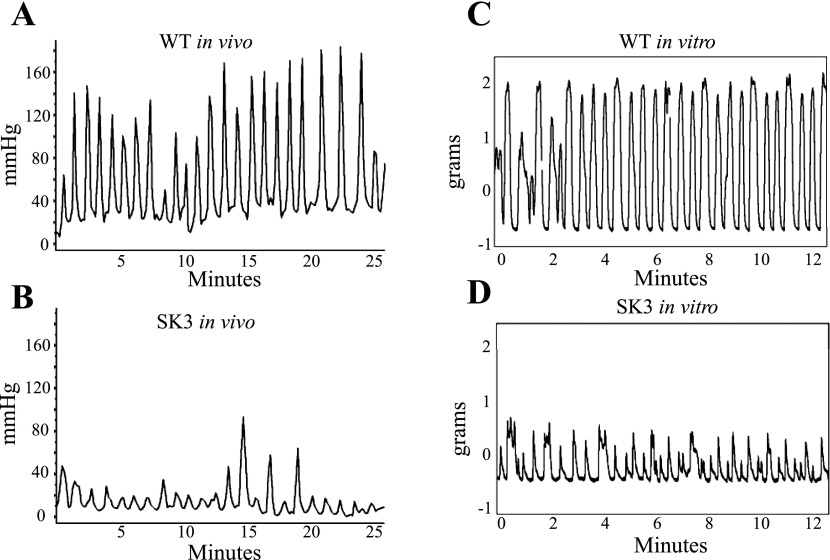
To determine whether intrauterine pressure can be measured during labor in mice, we implanted pressure transmitters into C57BL/6J WT mice on 8–9 dpc, as this resulted in optimal fetal outcome in these animals. Continuous measurement of pressure was initiated at 18 dpc and continued through the time of delivery, which is typically at ∼19.5 dpc (15). Because disturbing a dam during parturition can cause her to cease labor (9), mice were checked briefly near the beginning of each light cycle and just before each dark cycle to determine whether delivery had occurred. Pressure peaks visibly increased in magnitude and number at the time coinciding with labor and delivery (Fig. 2). Data output from this telemetry recording is provided as supplemental data (Supplemental Table S1 and Supplemental Fig. S1).1 SK3T/T mice were implanted similarly, but optimal fetal outcome was established on 13–14 dpc. Measurements of intrauterine pressure during delivery in WT and SK3T/T mice were compared. This revealed that the oscillations measured by telemetry for the WT and SK3T/T mice (Fig. 3, A and B, respectively) mimicked those observed by isometric tension in myometrial strips isolated from the same strains at 19 dpc (Fig. 3, C and D, respectively) (10). However, the in vivo pressure oscillations during parturition occurred at longer intervals than the force oscillations measured by the recording of isometric tension (Fig. 3, A and B, vs. Fig. 3, C and D). The observed similarities in the oscillations suggest that the intrauterine pressure readings are good indicators of uterine contractile activity. The differences in timing underscore the notion that in vitro measurements do not accurately reflect all aspects of these oscillations (perhaps because they are not subject to modification by endogenous factors that likely contribute to the oscillatory nature of pressure in the uterus).
Fig. 2.
Continuous recording of intrauterine pressure. The graph shows an example of wild-type (WT) intrauterine pressure beginning on 18.5 days postcoitum (dpc) and continuing through 20.5 dpc, revealing that spikes in intrauterine pressure increase in magnitude and frequency during labor. Alternating white and black bars above the graph indicate light and dark cycles, respectively. The lower black bar indicates the time of delivery.
Fig. 3.
Comparison of in vivo and in vitro recordings from mice during pregnancy. A: sample tracing of WT mouse intrauterine pressure recorded in vivo during delivery. B: tracing of SK3 channel-overexpressing (SK3T/T) mouse intrauterine pressure recorded in vivo during delivery, demonstrating that pressure waves are not produced as rapidly as in WT mice and that those produced are not of the same magnitude. C: representative isometric tension recording from myometrial strips of a 19 dpc WT mouse, demonstrating a wave pattern similar to that recorded by in vivo measurement. D: isometric tension recording from a 19 dpc SK3T/T uterus reveals that the frequency and strength of tension peaks are reduced, consistent with results from in vivo recordings.
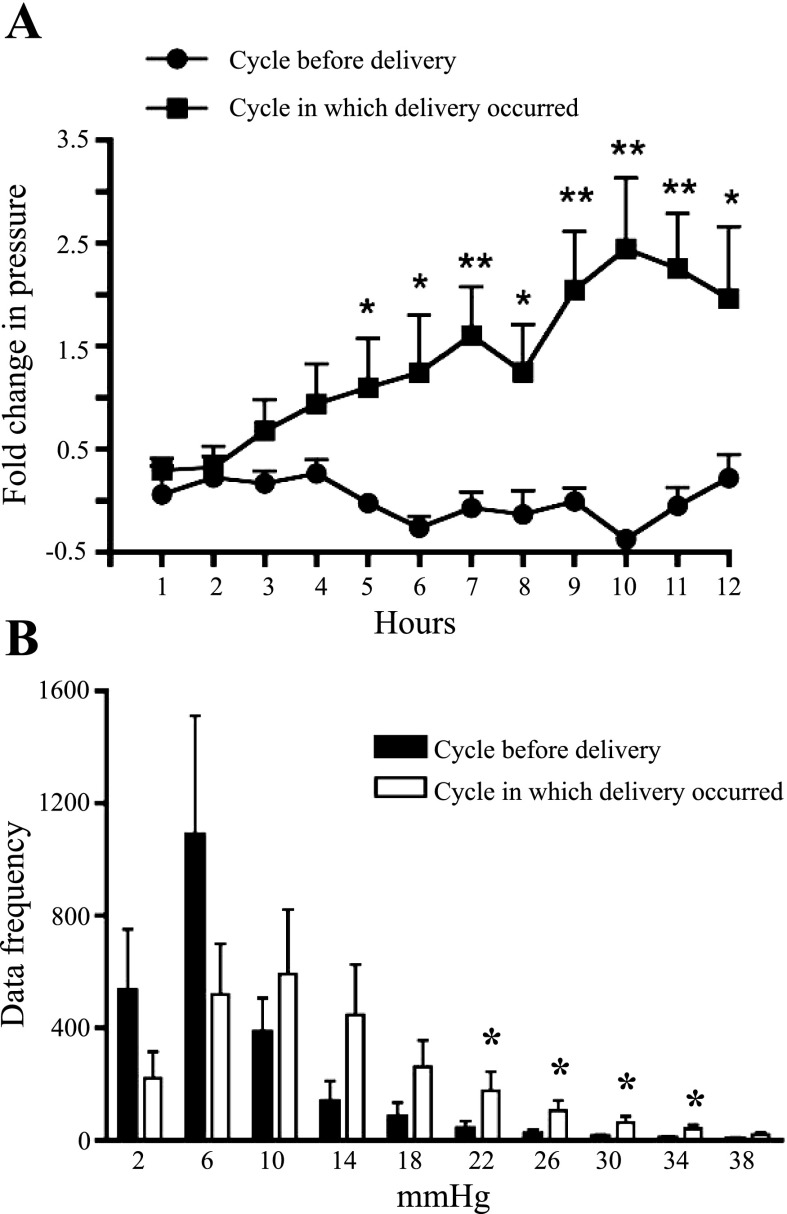
To better understand how pressures change during pregnancy, we measured average pressures (on an hourly basis) before and during pregnancy. The hourly averages calculated during the 12-h cycle in which the mouse delivered were much lower than the individual pressure spikes during delivery, evident in Fig. 2. However, the overall hourly average pressures during delivery were greater than baseline pressure, which was determined from readings during the 12-h cycle preceding delivery (Fig. 4). The average basal intrauterine pressure was 6.9 ± 1.4 mmHg during the light or dark cycle before the cycle in which delivery occurred. The average intrauterine pressures in the cycle during which delivery occurred increased gradually, peaking at 25.1 ± 5.6 mmHg, which is threefold greater than the maximal pressure during the preceding 12-h cycle (Fig. 4A).
Fig. 4.
Fold change in baseline intrauterine pressure, as measured by telemetry, in WT mice during pregnancy. A: an hourly moving average was calculated for both the 12 h before delivery (●) and the 12 h in which delivery occurred (■) (n = 7). B: histogram comparing the frequency of a given pressure for the 12-h cycle before delivery and for the 12-h cycle in which delivery occurred. Pressure shift to the right was seen in the 12 h during delivery (n = 7). Values are means ± SE. *P < 0.05, **P < 0.01.
Having determined, based on telemetry recordings, that average intrauterine pressure increases during delivery we next compared the shift in peak pressures generated during parturition more directly. We compared the frequency of the various pressures attained for the 12-h cycle before and the 12-h cycle during delivery. Using Dataquest A.R.T. 4.2 software (DSI), we were able to generate a histogram binned into 10 divisions of 4 mmHg each between pressures of 0 and 40 mmHg, in order to assess whether there was a shift to greater pressures in the cycle in which delivery occurred. As seen in Fig. 4B, the frequency distribution of the pressure during delivery shifted to the right, indicating that a greater number of peaks occurred at higher pressures during the cycle in which delivery occurred. This indicates that the transmitters can measure significant pressure changes in the uterus, consistent with labor.
Induced preterm labor leads to increased intrauterine pressure.
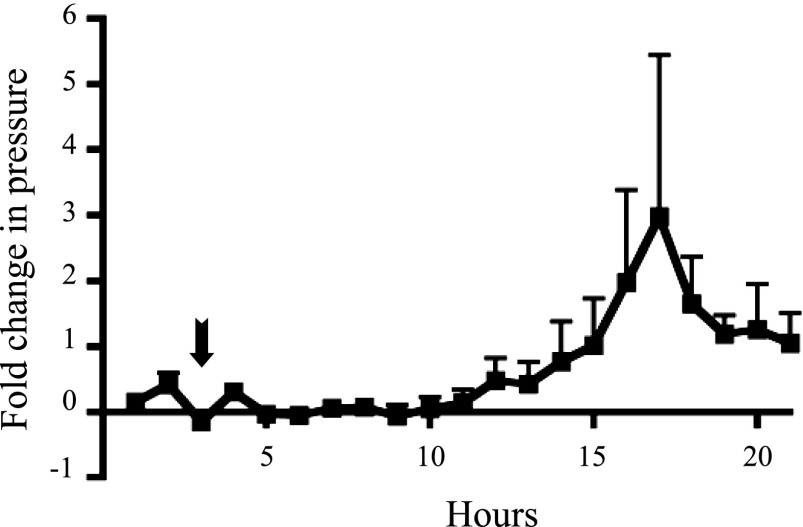
To determine whether radiotelemetry can be used earlier in gestation with a model of induced preterm delivery, we induced labor in WT mice by injecting 100 μg of RU486 on 15 dpc (10). Injection was carried out at 1:00 PM, and all mice delivered within 24 h of injection. Hourly averages demonstrated that intrauterine pressure consistently began to rise ∼8 h after injection (Fig. 5). Approximately 6 h later pressure peaked; this correlated with the delivery time frame found in previous studies after RU486 injection in mice (5). Uterine pressure returned to baseline after delivery. This experiment suggests that radiotelemetry can be utilized as early as 15 dpc in mice induced to undergo labor preterm.
Fig. 5.
Baseline intrauterine pressure (hourly average) during induced preterm labor in WT mice. Recordings began at 11:00 AM, and mice were injected with RU486 at 1:00 PM (indicated by arrow). Intrauterine pressure began to increase ∼8 h after injection and peaked 6 h later (n = 3).
Mouse model of delayed labor exhibits reduced intrauterine pressure during labor cycle.
Having successfully produced intrauterine recordings in WT mice, we wanted to investigate the novel information radiotelemetry could detect in a mouse model of disrupted parturition. We used SK3T/T mice that, in contrast to their WT counterparts, had the best fetal outcome when surgery was performed on 13–14 dpc. Recordings began on 18 dpc and continued until delivery or 22 dpc. Since most SK3T/T mice do not deliver on the appropriate gestational day, we compared the time periods that coincided with typical WT delivery. Uterine pressures in the SK3T/T mice remained near baseline and did not show an increase during the time frame consistent with WT delivery (Fig. 6). When SK3T/T mice were able to deliver pups, they did not exhibit the gradual increase in pressure seen during the course of delivery in WT mice. Although several SK3T/T mice produced intrauterine pressures above the baseline that was sufficient for delivery, they were usually not of the same magnitude as those of their WT counterparts (Fig. 7). The few SK3T/T mice that did exhibit pressures of the same magnitude as WT mice were not able to sustain these pressures, a deficiency that could have contributed to their failure to deliver in a time frame similar to that typical for WT mice (19.5 dpc).
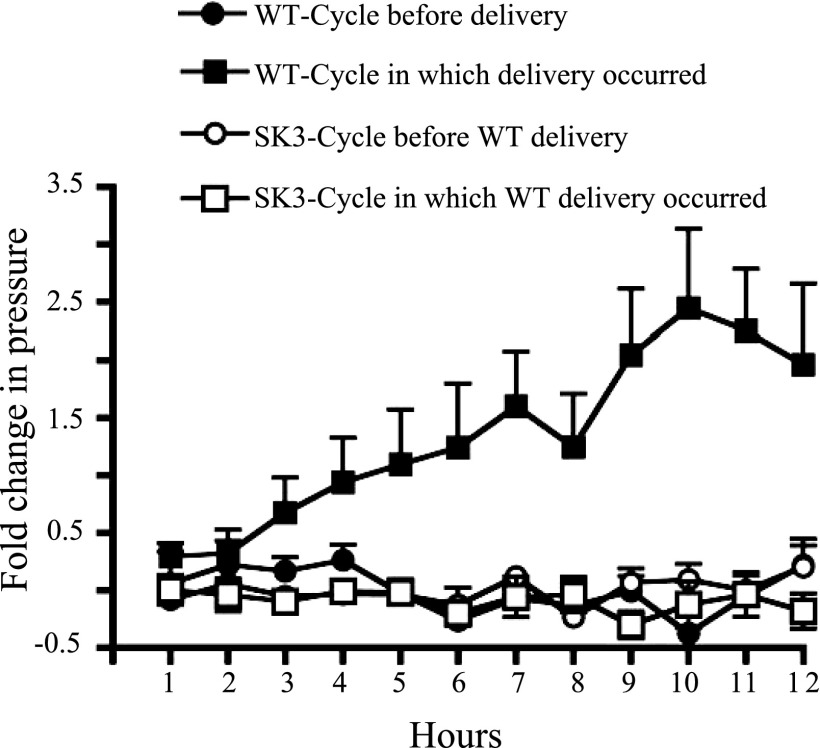
Fig. 6.
Baseline intrauterine pressure (hourly average) during pregnancy in SK3T/T vs. WT mice. In SK3T/T mice that were not able to deliver by 19.5 dpc, basal intrauterine pressure did not increase according to the dark cycle in which delivery occurred as it does in WT mice (WT data duplicated from Fig. 4). SK3T/T mice maintained an intrauterine pressure consistent with the baseline pressure seen in WT mice during the 12-h cycle before delivery (WT n = 7, SK3T/T n = 3).
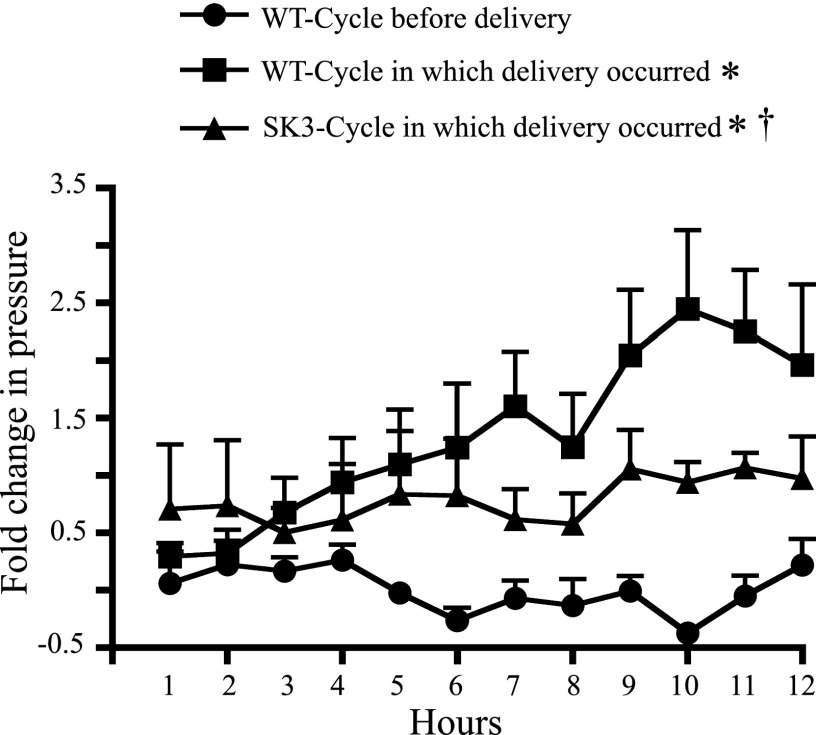
Fig. 7.
Hourly averages of SK3T/T mouse intrauterine pressure during delivery. Baseline intrauterine pressure (hourly average) increased above baseline in SK3T/T uteri during delivery, but not to the same extent as that seen during WT delivery (WT data duplicated from Fig. 4). Baseline intrauterine pressure in SK3T/T mice during delivery also was not gradually elevated as seen in WT mice during delivery. Values are means ± SE (WT n = 7, SK3 n = 4). *P < 0.001 vs. WT-cycle before delivery, †P < 0.05 vs. WT-cycle in which delivery occurred.
Power spectrum analysis of intrauterine pressure during prelabor and labor in WT and SK3T/T mice.
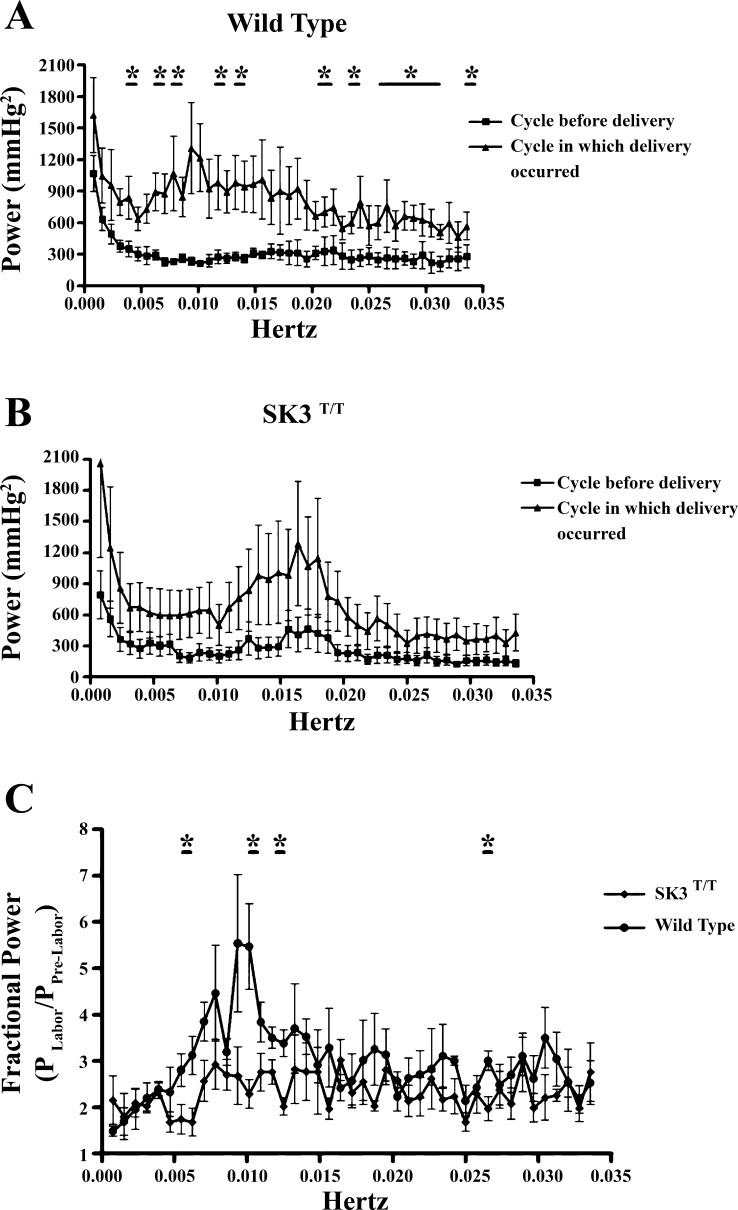
To identify the frequencies of the intrauterine pressure waves that change during the 12-h cycle in which delivery occurs, we performed fast Fourier transformations for the pressure recordings of the 12-h cycle preceding delivery and the 12-h cycle in which delivery occurred. In WT mice, the power spectrum of intrauterine pressure increased during the cycle in which delivery occurred relative to the power spectrum for the 12-h cycle before delivery (Fig. 8A). These increases in power were significantly greater in the case of contractile frequencies centered on 0.0038, 0.0065, 0.0161, 0.0238, 0.02778, and 0.0333 Hz. Although SK3T/T mice also exhibited an apparent increase in the power spectrum of intrauterine pressure during parturition, this difference was not significant given the variance between animals (Fig. 8B). When comparing the power spectrum of WT versus SK3T/T mice for the cycle of delivery, we found that WT mice generated significantly higher pressures, with frequencies of 0.0064, 0.0102, 0.0127, and 0.0263 Hz, compared with SK3T/T mice, whose power peaked at similar frequencies but not to the same extent (Fig. 8C). The rise in power of 0.005–0.010 Hz that occurred during WT labor was not produced during SK3T/T labor (Fig. 8C), indicating that contractions in that range of frequencies may be dampened by the overexpression of SK3 channels.
Fig. 8.
Power spectrum of intrauterine pressure during the 12-h cycle before delivery and the 12-h cycle in which delivery occurred. A: the power spectrum of WT baseline intrauterine pressure produced in the 12-h cycle during delivery increased above the baseline frequency measured during the 12-h cycle before delivery. Values are means ± SE (n = 4). *P < 0.05 for cycle before delivery vs. cycle in which delivery occurred. B: the power spectrum generated from SK3T/T intrauterine pressure during the 12-h cycle in which delivery occurred was not significantly elevated above that for the 12-h cycle before delivery. C: comparison of the normalized power spectra of WT and SK3T/T intrauterine pressure during the 12-h cycle in which delivery occurred, demonstrating that at certain frequencies more power was produced during WT delivery. Values are means ± SE (n = 4 for both WT and SK3T/T). *P < 0.05 vs. WT-cycle in which delivery occurred.
DISCUSSION
Most studies that investigate myometrial contractility have relied on in vitro measurements of tension. Such methods are useful, and will continue to be necessary for studying uterine contractions early in gestation (when the uterus contracts asynchronously) as well as for investigating the effects of drugs, toxins, and other solutions that cannot be administered to a live animal. However, in vivo approaches are necessary in some contexts, for example, the relationship of contractility to the progression of labor or the time at which labor begins. In addition, getting a complete picture of changes in uterine contractile pattern over the course of pregnancy through in vitro approaches would require sacrificing large numbers of animals. Furthermore, the in vitro approach requires that uterine strips are mounted either vertically or horizontally, which limits investigation of the contractions to those produced by either the circular or the longitudinal muscle layer of the uterus; as such, removing a section from a late-gestation uterus to study its contractility in vitro both changes contractile fiber orientation and fails to account for most endogenous neurohumoral influences. The use of uterine telemetry eliminates these obstacles, making it possible to more accurately study parturition in mouse models.
One mouse model that exhibits compromised parturition is the SK3T/T mouse that was used in this study (1, 10). Using an in vitro approach, we previously demonstrated (10) that uterine contractions in these mice were dampened, suggesting that altered uterine function leads to dysfunctional delivery. Our application of a more advanced methodology, radiotelemetric technology, enabled us to more accurately study the SK3 channel's contribution to myometrial contraction. We found that whereas intrauterine pressure gradually increased threefold during delivery in WT animals, in SK3T/T mice it failed to increase to a similar extent at the same stage of gestation. Furthermore, we demonstrated that excess SK3 channels resulted not only in dampening of uterine contractions but also in a reduction of the frequency of contraction in the cases when the mice did deliver. During delivery in SK3T/T mice, basal pressure did not increase gradually as in delivery in WT mice, indicating that even when these mice are able to produce pressures adequate for delivery, the overall elevation in basal pressure may not be sufficient to ensure consistent delivery on 19.5 dpc. This prior unknown function of SK3 channels in uterine activity would remain undiscovered in the absence of radiotelemetry. Thus studying uterine contraction in a live animal has given us the capacity to obtain more precise details about the mechanisms underlying parturition, and to extract new information that will provide insights into the process of labor.
Although radiotelemetry was successfully used in mice, one of the challenges that had to be overcome was fetal loss following implant of the telemetry transmitter. All transgenic and WT mice in this study had some degree of fetal loss in both uterine horns regardless of whether that horn was chosen for implant. Modifying the time of implantation according to strain made successful term pregnancies more consistent, but full litters were not produced. Given that intrauterine pressure in WT mice did not correlate with delivery of larger litters versus those that delivered smaller litters, it seems that the number of pups to be birthed does not affect overall intrauterine pressure. The fact that the optimal time of surgery for SK3T/T mice was different from that in WT mice suggests that modifications to the surgery protocol may be necessary for each mouse model of interest. Fetal loss following telemeter implant was not completely alleviated in either the WT or the mutant strain, but fetal outcome is expected to improve as the transmitter technology is refined. This will undoubtedly advance the usefulness of this technology in studies of the mechanisms that contribute to labor.
Our in vivo recordings demonstrate that intrauterine pressure measurements in the mouse yield data consistent with those obtained by in vitro tension measurements, indicating that this approach can be used to accurately quantify uterine activity. As in the telemetry-based study in rat uteri, we found that intrauterine pressure in WT mice increased at the end of pregnancy. We also discovered that not only the baseline pressure but also the number of contractions that produce higher pressure rises. Of further interest is the fact that the WT uterus showed a shift in the pressure produced during delivery compared with the previous 12-h cycle (Fig. 8), reaching a maximum of 189 ± 31 mmHg (Fig. 3). These findings illustrate that the information obtained from in vivo uterine recordings will provide useful new details about the genes that are targeted in mouse models of parturition, which will in turn lead to a better understanding of the mechanistic basis of labor and delivery in humans.
Genetic predisposition is one risk factor for preterm delivery (3, 11). As data from the Genome-Wide Association Study (GWAS) become available, multiple genes will be identified that may impact the relative risk of preterm labor. Subsequently, these genes can be targeted in mouse models in order to further study their function during parturition. Thus technological advances that allow more precise testing of myometrial contractions in mice are expected to greatly impact on the field of prevention and treatment of preterm labor by facilitating the identification of novel mechanisms underlying parturition.
Many genetically altered mice have been used to study single-gene disorders in which the regulation of parturition is defective. Notably, as pointed out by Mitchell and Taggart in a recent review (8), surprisingly little attention has been given to those mice that exhibit altered myometrial contraction. While these authors use this point to emphasize the need to understand how these mouse phenotypes relate to human parturition, we also must fully explore the mechanisms by which these models disrupt or accelerate the process of labor, even when the mechanism is presumed unrelated to uterine contraction. Thus the field would benefit greatly from an advanced technique, such as radiotelemetry, that makes it possible to measure intrauterine pressure in mice.
GRANTS
This work was supported by grants, from the March of Dimes (21-FY08-566) and the National Institute of Child Health and Human Development (HD-037831), awarded to S. K. England. S. L. Pierce is supported by a Predoctoral Fellowship from the American Heart Association (09PRE2280322).
DISCLOSURES
No conflicts of interest are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Kathryn Lamping of the Department of Internal Medicine at the University of Iowa for her critical review of the manuscript. The authors also thank the Kamal Rahmouni lab, the Mark Anderson lab, and the Curt Sigmund lab of the Department of Internal Medicine for sharing of equipment. We want to thank Data Sciences International for lending equipment and their technical support. We also thank Christine Blaumueller for editorial contributions to the manuscript.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- 1. Bond CT, Sprengel R, Bissonnette JM, Kaufmann WA, Pribnow D, Neelands T, Storck T, Baetscher M, Jerecic J, Maylie J, Knaus HG, Seeburg PH, Adelman JP. Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+ channel subunit, SK3. Science 289: 1942–1946, 2000. [DOI] [PubMed] [Google Scholar]
- 2. Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics 118: 1566–1573, 2006. [DOI] [PubMed] [Google Scholar]
- 3. DeFranco E, Teramo K, Muglia L. Genetic influences on preterm birth. Semin Reprod Med 25: 40–51, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Dudley DJ, Chen CL, Branch DW, Hammond E, Mitchell MD. A murine model of preterm labor: inflammatory mediators regulate the production of prostaglandin E2 and interleukin-6 by murine decidua. Biol Reprod 48: 33–39, 1993. [DOI] [PubMed] [Google Scholar]
- 5. Gonzalez JM, Xu H, Chai J, Ofori E, Elovitz MA. Preterm and term cervical ripening in CD1 mice (Mus musculus): similar or divergent molecular mechanisms? Biol Reprod 81: 1226–1232, 2009. [DOI] [PubMed] [Google Scholar]
- 6. Mackay LB, Shi SQ, Garfield RE, Maner WL. The effect of bilateral pelvic neurectomy on uterine and abdominal electrical and pressure activity, as measured by telemetry in conscious, unrestrained pregnant rats. J Perinat Med 37: 313–319, 2009. [DOI] [PubMed] [Google Scholar]
- 7. Mills PA, Huetteman DA, Brockway BP, Zwiers LM, Gelsema AJ, Schwartz RS, Kramer K. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J Appl Physiol 88: 1537–1544, 2000. [DOI] [PubMed] [Google Scholar]
- 8. Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol 297: R525–R545, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Newton N, Foshee D, Newton M. Experimental inhibition of labor through environmental disturbance. Obstet Gynecol 27: 371–377, 1966. [PubMed] [Google Scholar]
- 10. Pierce SL, Kresowik JD, Lamping KG, England SK. Overexpression of SK3 channels dampens uterine contractility to prevent preterm labor in mice. Biol Reprod 78: 1058–1063, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plunkett J, Muglia LJ. Genetic contributions to preterm birth: implications from epidemiological and genetic association studies. Ann Med 40: 167–195, 2008. [DOI] [PubMed] [Google Scholar]
- 12. Ratajczak CK, Muglia LJ. Insights into parturition biology from genetically altered mice. Pediatr Res 64: 581–589, 2008. [DOI] [PubMed] [Google Scholar]
- 13. Shi SQ, Maner WL, Mackay LB, Garfield RE. Identification of term and preterm labor in rats using artificial neural networks on uterine electromyography signals. Am J Obstet Gynecol 198: 235.e231–235.e234, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simhan HN, Caritis SN. Prevention of preterm delivery. N Engl J Med 357: 477–487, 2007. [DOI] [PubMed] [Google Scholar]
- 15. Werboff J, Anderson A, Haggett BN. Handling of pregnant mice: gestational and postnatal behavior effects. Physiol Behav 3: 35–39, 1968. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.