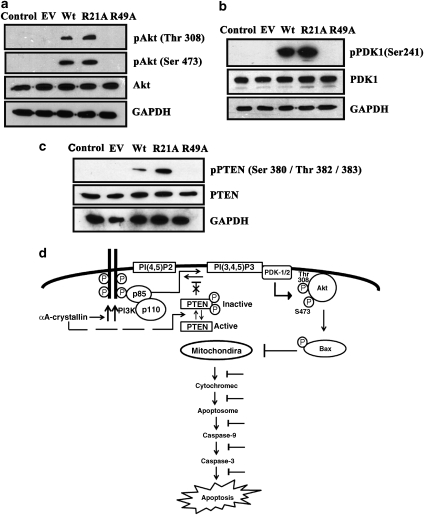
Figure 6.
R21A induces Akt, PDK1 and phosphatase tensin homologue (PTEN) phosphorylation. HeLa cells overexpressing human Wt, R21A or R49A αA-crystallin were treated with 100 nM staurosporine or 0.01% dimethyl sulfoxide (DMSO) for 12 h. Cell lysates (100 μg of protein each) were subjected to western blot analysis. (a) pAkt levels at Thr308 and Ser473 using an anti-pAkt (Thr308) and anti-pAkt (Ser473) polyclonal antibodies, respectively. Total Akt was detected using an anti-Akt polyclonal antibody. (b) Detection of pPDK1 and total PDK1 using an anti-pPDK1(Ser241) and anti-PDK1 polyclonal antibodies, respectively. (c) Detection of pPTEN (Ser380/Thr382/383) and total PTEN using an anti-pPTEN polyclonal and an anti-PTEN polyclonal antibody. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as loading control. (d) A conceptual mechanism by which αA-crystallin inhibits apoptosis by the PI3K/PDK1/Akt pathway: αA-crystallin inhibits apoptosis by the PI3K/PDK1/Akt pathway. αA-crystallin enhances the PI3K activity and decreases PTEN activity through phosphorylation (dashed line) leading to increased production and retention of phosphatidylinositol(3,4,5)-triphosphate (PI(3,4,5)P3). This favors recruitment of Akt to the plasma membrane allowing its phosphorylation at Thr308 and Ser473 by PDK1/2. Activated Akt then detaches from the membrane and inactivates Bax by phosphorylation. Inactive Bax is unable to translocate to mitochondria, which prevents cytochrome c release into the cytoplasm. The ensuing inhibition of caspase activation leads to inhibition of apoptosis