Abstract
Six intraoperative blood samples were obtained at intervals from each of 100 individuals undergoing their first liver transplants. The patients fell into the following diagnostic categories: postnecrotic cirrhosis 28, primary biliary cirrhosis 20, sclerosing cholangitis 19, miscellaneous diseases 14, carcinoma/neoplasia 12 and fulminant hepatitis 7. Coagulation factor values in the initial (baseline) blood samples varied by patient diagnosis. In general, all factor levels were reduced except factor VIII:C, which was increased to almost twice normal. The slight intraoperative changes in factors II, VII, IX, X, XI and XII suggested that a steady-state relationship existed between depletion (consumption/bleeding) and repletion (transfusion, transit from extra- to intravascular space), even in the anhepatic state. In contrast, there were rapid and very significant falls in factor VIII and fibrinogen and a less pronounced decrease in factor V, all reaching their nadirs in early to mid-Stage III. The cause of these coagulation changes appears to be activation of the fibrinolytic system.
Liver transplantation has been used to treat end-stage liver disease caused by a wide variety of congenital or acquired disorders. Improved methods for procurement and preservation of the donor livers, innovative surgical techniques and improved immunosuppressive agents have made liver transplantation feasible for many patients with severely damaged liver parenchyma, vasculature or bile ducts. However, one of the major difficulties has been the need for multiple blood transfusions, which reflects the large loss of blood during the operation. This study deals with intraoperative changes in coagulation parameters and the quantities of red blood cells (RBC) transfused.
MATERIALS AND METHODS
Patients
During an approximate 2-year period, intraoperative coagulation studies were carried out on 100 adult individuals undergoing their first liver transplants. All of these transplants were done with an axillofemoral venous bypass using tubing with a heparinized surface (ARGYLE® tubing) which shunted the major blood flow away from the operative area. No patient received systemic heparin. All patients were transfused with a premixed “cocktail” of equal volumes (250 ml) of RBC, fresh-frozen plasma and Plasmalyte A®, which contained 526 mg per 100 ml sodium chloride, 502 mg per 100 ml sodium gluconate, 368 mg per 100 ml sodium acetate trihydrate, 37 mg per 100 ml potassium chloride, 30 mg per 100 ml magnesium chloride adjusted with sodium hydroxide to pH range 6.5 to 8.0. Previous testing of this mixture had shown the following mean values: hematocrit 27%, factor I (fibrinogen) 0.37 unit per ml, factor II (prothrombin) 0.59 unit per ml, factor V 0.21 unit per ml, factor VII 0.58 unit per ml and factor VIII 0.57 unit per ml (1). Transfusions of platelets and cryoprecipitate were usually started in Stage III. Intraoperative coagulation studies were performed at the request of the anesthesiologist or surgeon who had informed the patient that these would be necessary for regulation of therapy (plasma, RBCs, platelets, etc.).
The patients were categorized by pathological diagnosis as previously described (2). An additional diagnostic group, fulminant hepatitis, was included. The 100 patients fell into the six diagnostic groups shown in Table 1. The largest group comprised 28 patients with postnecrotic cirrhosis (PNC) and included patients with chronic active hepatitis, lupoid hepatitis or cryptogenic cirrhosis. The second largest group contained 20 female patients with primary biliary cirrhosis (PBC). An additional 19 patients had primary sclerosing cholangitis (SC). In the carcinoma/neoplasia (CA) group, eight patients had hepatomas, one of whom had preexisting cirrhosis secondary to congenital tyrosinemia. Three patients had angiosarcoma, and one patient, with a metastatic tumor of unknown primary site, was thought to have a vascular hepatic tumor preoperatively.
Table 1.
Diagnostic categories of 100 patients undergoing first liver transplants
| No. | Female | Male | Age range (years) |
|
|---|---|---|---|---|
| PNC | 28 | 14 | 14 | 16–52 |
| PBC | 20 | 20 | 0 | 27–54 |
| SC | 19 | 7 | 12 | 23–54 |
| MIS | 14 | 6 | 8 | 16–47 |
| CA | 12 | 8 | 4 | 21–54 |
| FUL | 7 | 0 | 7 | 27–57 |
| Total | 100 | 55 | 45 | 16–57 |
In the smallest group, containing seven patients with fulminant hepatitis (FUL), three were presumed to be infected with non-A, non-B hepatitis, one was infected with hepatitis B, two were secondary to hepatotoxins (gold and 2-nitropropane) and one was of unknown etiology.
A final miscellaneous (MIS) group included 14 patients with various diagnoses. Three patients had Wilson's disease, and there was one patient with each of the following diagnoses: α1-antitrypsin deficiency, hemachromatosis, Budd-Chiari syndrome, cirrhosis secondary to cystic fibrosis, polycystic disease of the liver, secondary biliary cirrhosis, congenital hepatic fibrosis, variant Caroli's disease (secondary biliary cholangiectasis), acquired hepatocerebral degeneration and cryptococcal cholangitis; a final patient had bile duct obstruction and cholangitis secondary to hepatic trauma.
Coagulation Tests
The tests performed employed previously published methods (3–5) and included prothrombin time (PT), activated partial thromboplastin time (APTT), euglobulin lysis time (ELT) and assays of coagulation factors I, II, V, VII, VIII, IX, X, XI and XII. Fibrinogen was expressed as units per ml by dividing the mg per dl by 300 mg per dl (the normal value for this laboratory). Commercially obtained standards (lyophilized) and locally produced frozen pooled plasma were tested with each group of assays (approximately every 2 hr). All blood samples for these tests were chilled immediately, centrifuged in the cold and assayed as rapidly as possible. The euglobulin lysis time is a crude but rapid screening test for the presence of fibrinolytic activity. The euglobulin fraction used in the test contains most of the activator, plasminogen and fibrinogen in the original plasma. The actual lysis time is dependent upon the concentrations of these factors and any traces of inhibitors which might be coprecipitated.
For various reasons, not all coagulation tests were done on all samples (see sample numbers in parentheses, Table 2). The PT and APTT were done on all six samples from 55 patients; the ELT, fibrinogen and factor X were performed on six samples from 99 patients. Other factors were assayed on the six samples from the 100 patients.
Table 2.
| Sample comparisons |
Test (no. of specimens) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|||||||||
| PT | APTT | ELT | II | V | VII | X | I | IX | XI | XII | VIII | |
| (330) | (330) | (594) | (600) | (600) | (600) | (594) | (594) | (600) | (600) | (600) | (600) | |
| 1–2 | NSb | NS | 0.00001 | NS | 0.01 | NS | NS | 0.01 | NS | NS | NS | NS |
| 1–3 | NS | NS | 0.00001 | NS | 0.00001 | NS | 0.01 | 0.00001 | NS | NS | NS | 0.00001 |
| 1–4 | 0.01 | 0.00001 | 0.00001 | 0.00001 | 0.00001 | 0.0001 | 0.00001 | 0.00001 | 0.00001 | NS | 0.001 | 0.00001 |
| 1–5 | NS | 0.01 | NS | 0.00001 | 0.00001 | 0.01 | 0.00001 | 0.00001 | 0.00001 | NS | 0.001 | 0.00001 |
| 1–6 | NS | NS | NS | 0.01 | 0.00001 | NS | 0.00001 | 0.00001 | NS | NS | NS | 0.00001 |
| 2–3 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | 0.00001 |
| 2–4 | 0.001 | 0.00001 | NS | 0.0001 | 0.00001 | 0.01 | 0.00001 | 0.0001 | 0.00001 | NS | 0.01 | 0.00001 |
| 2–5 | 0.01 | 0.001 | 0.01 | 0.0001 | 0.00001 | NS | 0.00001 | 0.0001 | 0.0001 | NS | 0.01 | 0.00001 |
| 2–6 | NS | NS | 0.00001 | NS | 0.00001 | NS | 0.00001 | 0.01 | NS | NS | NS | 0.00001 |
| 3–4 | 0.001 | 0.00001 | NS | 0.001 | 0.00001 | NS | 0.00001 | NS | 0.0001 | NS | NS | 0.00001 |
| 3–5 | 0.01 | 0.001 | 0.00001 | 0.01 | 0.00001 | NS | 0.00001 | NS | NS | NS | NS | 0.00001 |
| 3–6 | NS | NS | 0.00001 | NS | 0.00001 | NS | NS | NS | NS | 0.01 | NS | 0.00001 |
| 4–5 | NS | 0.01 | 0.00001 | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| 4–6 | NS | 0.00001 | 0.00001 | 0.01 | NS | NS | NS | NS | 0.00001 | 0.001 | NS | NS |
| 5–6 | NS | 0.001 | 0.01 | 0.01 | NS | NS | 0.01 | NS | 0.01 | NS | NS | NS |
Alpha or p value for the significance of differences among the means of coagulation tests at different stages in liver transplantation. The outer column of sample numbers indicates the sample value which is being compared to that in the second list.
NS = not significant.
Blood Sample Timing
Because the coagulation tests were performed as part of patient care and not as a research project, the timing varied somewhat from patient to patient. Each operation took from 6 to 12 hr or more. Stage I was the operative stage in which the diseased liver was dissected and removed and the site for the new liver was prepared; it lasted for 2 to 5 hr. Stage II was the time during which the liver was separated from the circulation and removed from the body—the anhepatic period; it usually lasted about 2 hr. Stage III was the period during which the new liver was inserted into the prepared bed, the circulation was established and bile ducts were revised. This took from 4 to 6 hr.
Multiple serial samples were taken during each operation at times when the anesthesiologist or surgeon anticipated problems. Only the six which fitted into the pattern shown in Table 3 were used in this study. If two or more samples fell within the same time period, the test results were averaged.
Table 3.
Blood sample timing patterns
| Stage | Sample no. |
Average time (hr) from skin incision |
Period |
|---|---|---|---|
| I | 1 | 0 | Initial or baseline |
| 2 | 3.5 | Late in Stage I (following dissection of diseased liver) | |
| II | 3 | 5.5 | Mid- to late Stage II (an-hepatic period) |
| III | 4 | 6.4 | Early in Stage III (restoration of circulation to the new graft liver) |
| 5 | 8 | Mid-Stage III | |
| 6 | 10 | End of operation |
Blood Use
The number of units of RBCs used are the figures compiled by Central Blood Bank (number of units released to patient minus number returned) and may differ slightly from the records of the anesthesiologists.
Plot Patterns
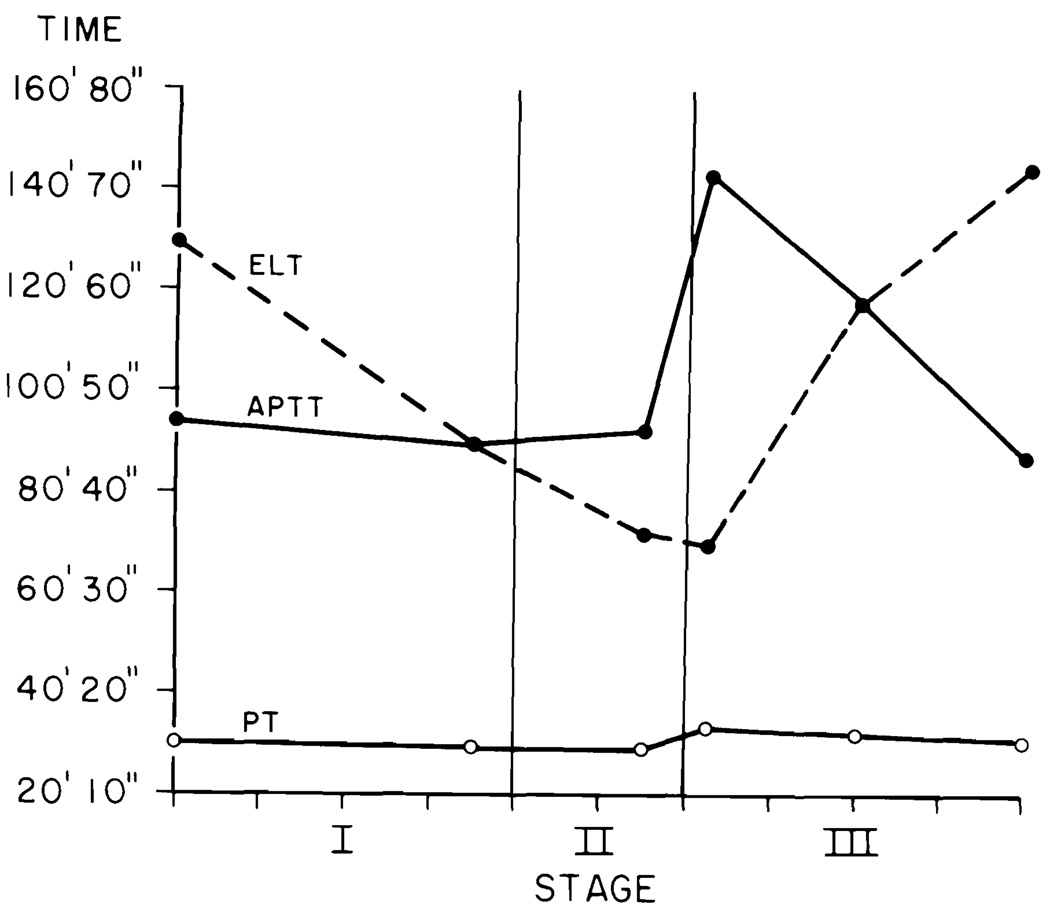
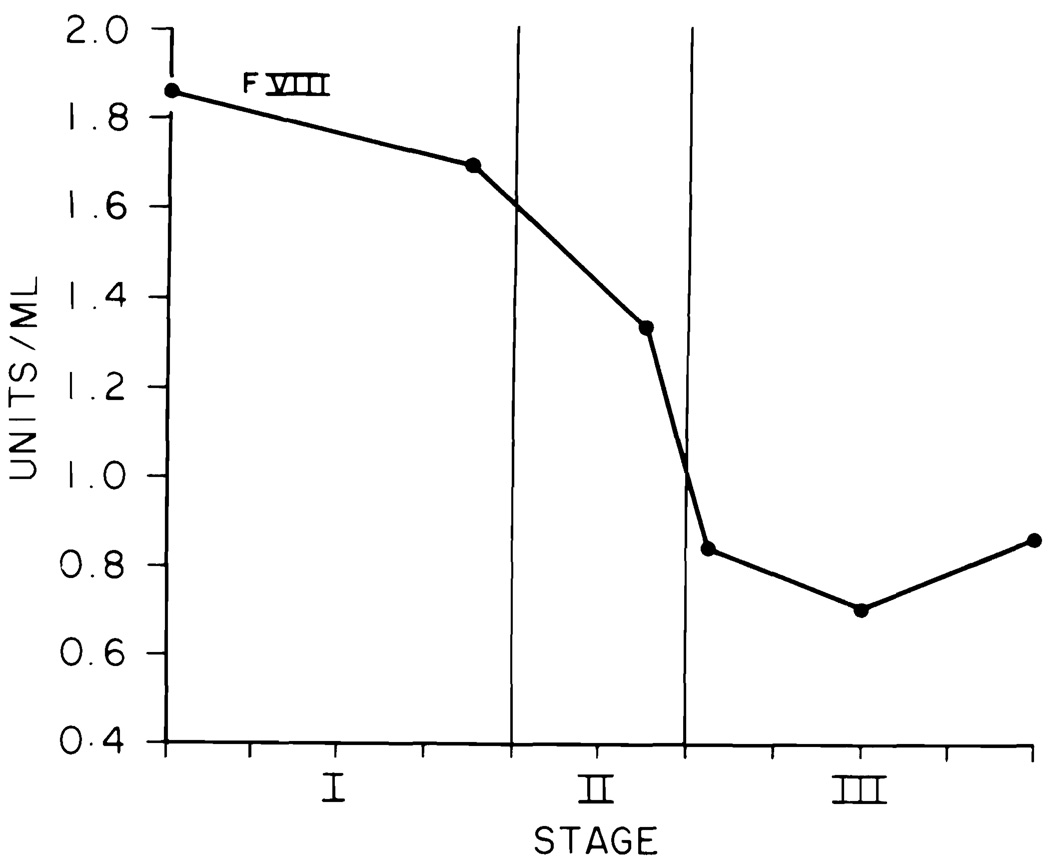
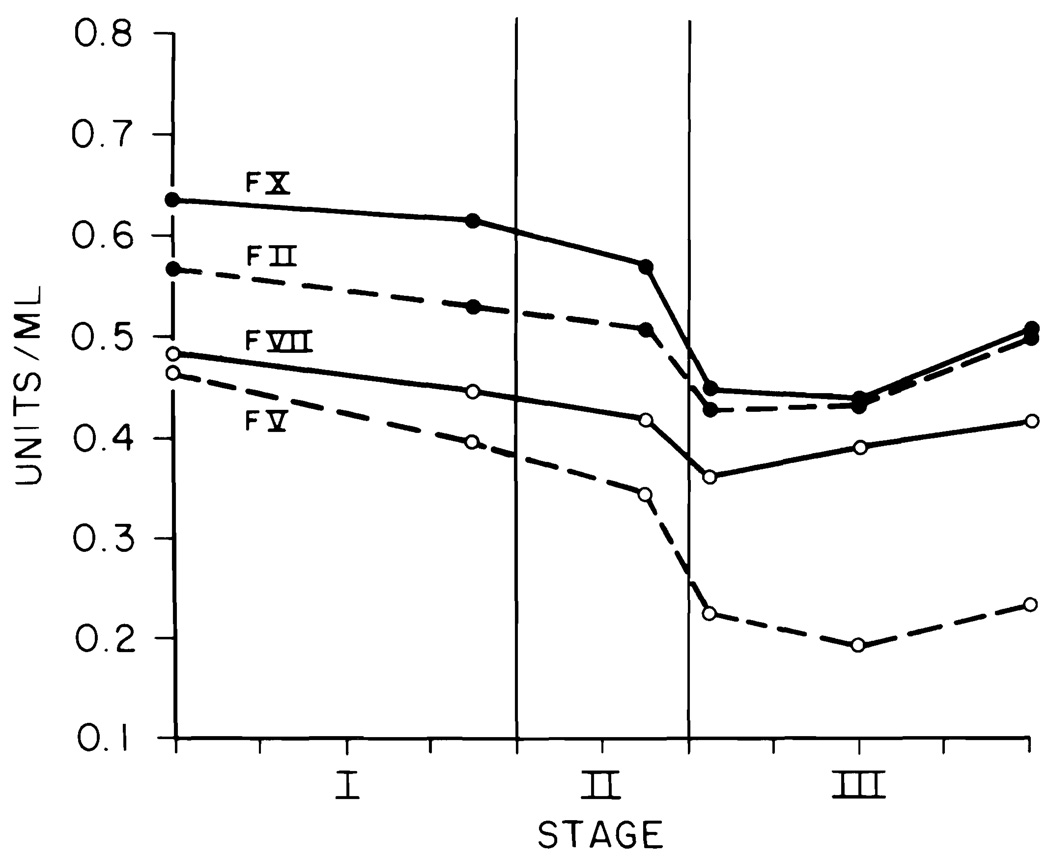
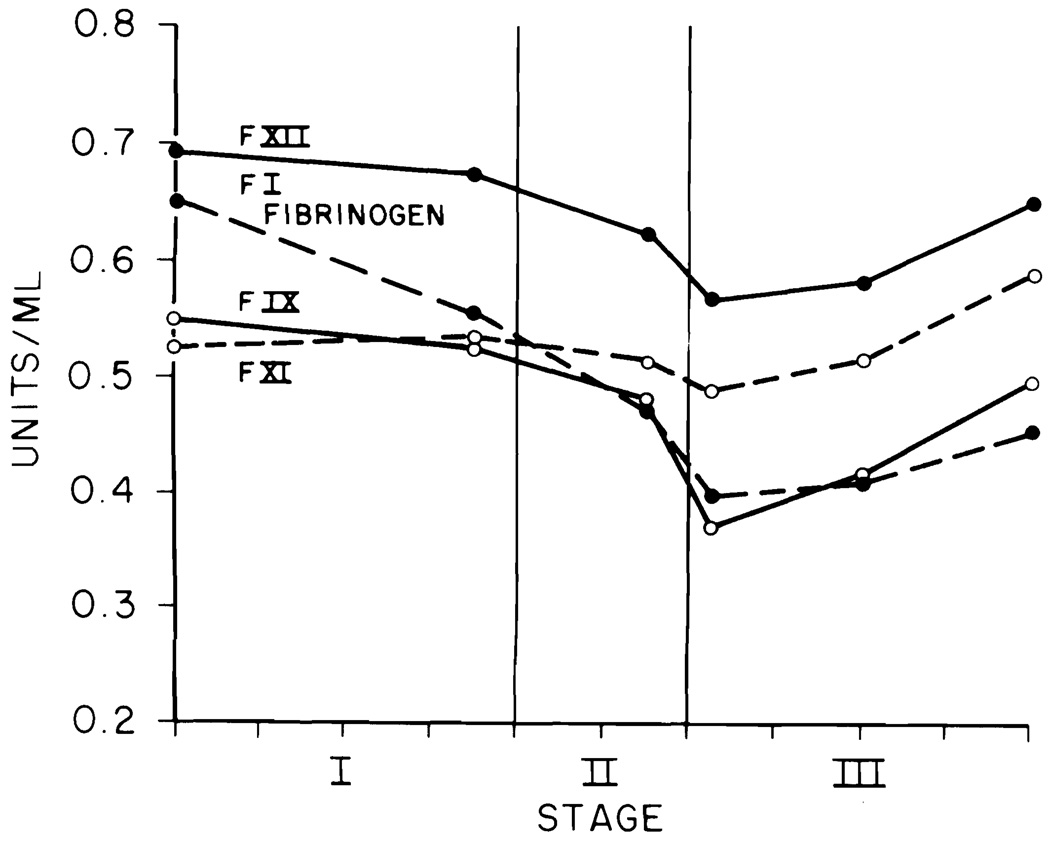
The data for each liver transplant were fitted into the blood sample timing pattern (Figs. 1 to 4). The stage of the operation is indicated on the abscissa. The mean duration of the 100 transplants was 10 hr. Each tick on the abscissa indicates 1 hr. The ordinate for Figure 1 gives the PT and APTT in sec and the ELT in min. The ordinates in Figures 2 to 4 are in units per ml.
Fig. 1.
Prothrombin time (PT) (sec), activated partial thromboplastin time (APTT) (sec) and euglobulin lysis time (ELT) (min).
Fig. 4.
Factor VIII (note scale change).
Fig. 2.
Factors II, V, VII and X.
Statistical Analysis
Analysis of variance was calculated on intraoperative data using the Statistical Analysis System's general linear model [p. 434 of Ref. (6)], in which t tests were performed on intraoperative means to calculate least significant differences. Only confidence levels of 0.01 or less were considered significant. Relationships between ELT, blood use and factor levels used ANOVA [p. 609 of Ref. (6)], a standard analysis of variance in addition to the nonparametric performance of ranks.
RESULTS
Figures 1 to 4 present means of the coagulation tests and factors, and Table 2 shows the alpha or p values for the significance of the difference for each mean from the others. In Table 2, the numbers in parentheses after the name of the test indicate the number of values used in the calculation. For example, for PT only 330 points were used. This means that only 55 patients had PT values for each timed sample, whereas factor II calculations used 600 points, indicating that all 100 patients had the six needed results.
Prothrombin Time and Activated Partial Thromplastin Time
PT and APTT are plotted on the seconds time scale of Figure 1. Both are abnormal in Sample 1. The PT curve is nearly flat and only Point 4 is significantly different from the others. The APTT curve shows a sharp, highly significant rise at Points 4 and 5, returning to normal at the end of the operation.
Eugloblin Lysis Time
The ELT on the initial sample (Fig. 1) was within the normal range (>120 min) in 78% of the 99 patients tested. Mildly activated initial lysis times were found in 14/28 (50%) of the PNC group, 6/14 (43%) of the MIS group, 1/12 (8%) of the CA group and 1/7 (14%) of the FUL group. None of the baseline studies on PBC or SC patients showed activated ELT times. Figure 1 shows that the ELT times decreased rapidly and were very significantly different from baseline on Samples 2, 3 and 4. By the middle of Stage III and end of operation, the ELTs had returned to normal.
Factors II, V, VII and X
Inspection of Figure 2 shows that the time curve for all four factors followed very similar patterns. The major decline in activity started late in the anhepatic stage and reached its nadir at Sample 4. Samples 5 and 6 showed minor recoveries in factors II, VII and X but essentially no recovery in factor V. All of the intraoperative factor V assays were below the baseline value and there was little or no recovery. Factor X's baseline assay was higher than that of the other factors, making the fall at Sample 4 appear more precipitous.
Factors I, IX, XI and XII
Fibrinogen and factor IX (Fig. 3) started to fall with the second sample and showed a poor recovery by the end of the operation. The curve for factor XI was almost flat. For factor XII there was a moderately significant decline from Sample 1 to Sample 4 with recovery in Samples 5 and 6.
Fig. 3.
Factors I (fibrinogen), IX, XI and XII.
Factor VIII
Factor VIII levels were plotted on a separate chart (Fig. 4) to allow inclusion of the initial very high level and the steep decline during the anhepatic stage and early Stage III. There was no significant recovery at the end of the procedure.
Blood Use
The mean RBC use during these 100 operations was 26.3 units, and the range was from 4 to 157 units.
Relationships between Coagulation Factor Rank, ELT and RBC Use
In order to determine whether or not the coagulation factor levels were dependent on the presence or absence of active fibrinolysis or were a determinant in the amount of blood used, the lowest levels of each were ranked. The lowest level was determined by picking (by computer) the least value of the factor on Samples 3, 4 or 5. These values were then ranked from 1 to 99 or 100. The first and second ELT columns of Table 4 show the mean ELT values for the lowest 20 and highest 79 patients ranked for fibrinogen. These are obviously different in comparison to the next line, factor II, which are almost the same for the low 20 and high 79. Factors VIII, XII, V and XI are dependent to a significant extent on the ELT. The last section of Table 4 shows the mean numbers of RBC units used in the low-rank 20 patients and the high-rank 80. This indicates that low levels of factor VIII, in particular, correlate with high use of RBCs. Fibrinogen, factor IX, factor XII and ELT to a lesser extent correlate with blood use.
Table 4.
Relationship between coagulation factor rank (low 20 and high 79 or 80), ELT score and RBC usea
| Factor | ELT score |
RBC units |
||||
|---|---|---|---|---|---|---|
| Low 20 |
High 79 |
p | Low 20 |
High 80 |
p | |
| FIBb | 1.8 | 2.59 | 0.007 | 43 | 22 | 0.0033 |
| II | 2.4 | 2.44 | NS | 32 | 25 | NS |
| V | 1.95 | 2.56 | 0.04 | 29 | 26 | NS |
| VII | 2.05 | 2.53 | NS | 21 | 28 | NS |
| VIII | 1.70 | 2.62 | 0.0016 | 60 | 18 | 0.0001 |
| IX | 2.10 | 2.52 | NS | 40 | 23 | 0.02 |
| X | 2.35 | 2.46 | NS | 23 | 27 | NS |
| XI | 1.95 | 2.56 | 0.04 | 35 | 24 | NS |
| XII | 1.90 | 2.57 | 0.02 | 39 | 23 | 0.02 |
| ELT | 1.0 | 2.78 | 0.0001 | 38 | 23 | 0.04 |
ELT score = 1 for 15 min, 2 for 30 min, 3 for 45 min, and 4 for 60 min or more.
FIB = fibrinogen (factor I).
DISCUSSION
Several points may be made by the analysis of the coagulation tests and factor levels during liver transplantation. The PT and APTT lengthen significantly during liver transplantation. The PT and APTT lengthen significantly at Sample 4 (early Stage III) and return toward normal by the end of the operation. The coagulation factor assays follow the same pattern. Factors II, VII, X, IX and XII fall during the anhepatic period, reaching their nadirs early in Stage III, and return to or toward the baseline value by the end of the operation. Factors I, V and VIII start to fall somewhat earlier and have made lesser recoveries by the end of the surgery. If the factor starts at below-normal levels, which is often the case when the liver parenchymal cells are damaged, a correction may be seen in the first hours, undoubtedly due to the infusion of fluids containing factors at higher levels than those circulating in the recipient. During the anhepatic phase, when there is little or no synthesis of most coagulation factors, highly significant declines, except in factor XI, are observed. For factor XI, we must presume that inflow from infusion and extravascular areas and, possibly, a slower ratio of metabolic breakdown due to long (24 to 48 hr) half-life, almost balance the outflow due to blood loss and lack of synthesis.
The activation of fibrinolysis and its effects on coagulation factor levels were studied by ranking the intraoperative levels of each factor into two groups: the lowest 20 and the highest 79 (Table 4). The mean ELT number, indicating extent of activation of ELTs in each group, was determined. The relationships between activation of fibrinolysis and decreases in factors VIII and I are highly significant and in factors V, XI and XII are slightly significant. Thus, the sequence of events appears to be activation of fibrinolysis in late Stage II followed by destruction of factors I and VIII with concomitant elevation of the APTT early in Stage III. Factors V, XI and XII also seem susceptible to fibrinolytic destruction.
Fibrinolysis may be activated by release of tissue plasminogen activator from vascular endothelium or possibly by inhibition of tissue plasminogen activator inhibitor by activated Protein C. It is theorized that in either case active plasmin is formed in quantities sufficient to destroy the susceptible coagulation factors (VIII, V and I).
Previous investigations of coagulation during canine and/or human liver transplantation and in human liver disease have been alternatively interpreted to show strong evidence for activation of fibrinolysis (7, 8), for disseminated intravascular coagulation (DIC) (9–12) or for both (13).
DIC (14) causes consumption of multiple coagulation factors, antithrombin III and platelets and the appearance of high levels of fibrin monomers, fibrin split products and microthrombi (7). In an early study of human and canine graft livers, the presence of microthrombi could not be demonstrated (10). This, however, does not rule out DIC since microthrombi could be lysed in situ. In addition, the limited falls in the coagulation factors and the lack of high levels of fibrin split products (15) indicate that DIC is mild or absent. Recently presented but unpublished data from our own laboratory show no significant change in antithrombin III during liver transplantation. These findings do not support the contention that DIC is the major cause of the changes observed during liver transplantation. A fibrinolytic process is more consistent with the findings.
The clinical importance of rapid and abrupt decline in factor VIII values is emphasized by the highly significant relationship between blood use and rank by lowest factor VIII value. Operations in which factor VIII fell into the lowest 20% used three times as much blood as did the 80% with higher factor VIII levels.
Recent thromboelastographic evidence (15), as well as these observations, support fibrinolysis as the predominant mechanism involved. Therapeutic intervention to counteract fibrinolysis (Amicar® and Cyklokapron®) may help to clarify its role and lead to prevention of life-threatening hemorrhage. Cryoprecipitate and/or heat-treated or virus-free factor VIII concentrates are available for treatment of the factor VIII deficiency.
Our results indicate that the most informative tests are PT, APTT, ELT and factor VIII level performed six to eight times (at 2- to 3-hr intervals) during the surgery. Platelet counts are also recommended to guide platelet replacement therapy.
Acknowledgment
The authors thank Mary Kay George for manuscript preparation.
REFERENCES
- 1.Kang YG. Monitoring and treatment of coagulation. In: Winter PM, Kang YG, editors. Hepatic transplantation: anesthetic and perioperative management. New York: Praeger Publishers; 1986. pp. 151–173. [Google Scholar]
- 2.Bontempo FA, Lewis JH, Van Thiel DH, et al. The relation of preoperative coagulation findings to diagnosis, blood usage, and survival in adult liver transplantation. Transplantation. 1985;39:532–536. doi: 10.1097/00007890-198505000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis JH. Coagulation defects. JAMA. 1961;178:1014–1020. doi: 10.1001/jama.1961.73040490010008. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JH. Hemostasis and hemorrhage. Sci Clin. 1971;1:l–66. [Google Scholar]
- 5.Lewis JH, Spero JA, Hasiba U. Bleeding disorders. Garden City, New York: Medical Examination Publishing Co., Inc.; 1978. Diagnostic methods: laboratory tests; pp. 22–34. [Google Scholar]
- 6.SAS Institute, Inc. SAS® user's guide: statistics, version 5 edition. Cary, North Carolina: SAS Institute, Inc.; 1985. [Google Scholar]
- 7.von Kaulla KN, Kaye H, von Kaulla E, et al. Changes in blood coagulation before and after hepatectomy or liver transplantation in dogs and man. Arch Surg. 1966;92:71–79. doi: 10.1001/archsurg.1966.01320190073016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boks AL, Brommer EJP, Schalm SW, et al. Hemostasis and fibrinolysis in severe liver failure and their relation to hemorrhage. Hepatology. 1986;6:79–86. doi: 10.1002/hep.1840060115. [DOI] [PubMed] [Google Scholar]
- 9.Boehmig HL, Fritsch A, Kux M, et al. Gerinnungsveranderungen bei orthotoper lebertransplantation am hund. Thromb Diath Haemorrh. 1969;21:332–335. [PubMed] [Google Scholar]
- 10.Hutchison DE, Genton E, Porter KA, et al. Platelet changes following clinical and experimental hepatic homotransplantation. Arch Surg. 1968;97:27–33. doi: 10.1001/archsurg.1968.01340010057003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pechet L, Groth CG, Daloze PM. Changes in coagulation and fibrinolysis after orthotopic canine liver homotransplantation. J Lab Clin Med. 1969;73:91–102. [PubMed] [Google Scholar]
- 12.Blecher TE, Terblanche J, Peacock JH. Orthotopic liver homotransplantation. Arch Surg. 1968;96:331–339. doi: 10.1001/archsurg.1968.01330210009003. [DOI] [PubMed] [Google Scholar]
- 13.Groth CG, Pechet L, Starzl TE. Coagulation during and after orthotopic transplantation of the human liver. Arch Surg. 1969;98:31–34. doi: 10.1001/archsurg.1969.01340070049006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spero JA, Lewis JH, Hasiba U. Disseminated intravascular coagulation: findings in 346 cases. Thromb Diath Haemorrh. 1980;43:28–33. [PubMed] [Google Scholar]
- 15.Kang YG, Martin DJ, Marquez J, et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth Analg. 1985;64:888–896. [PMC free article] [PubMed] [Google Scholar]