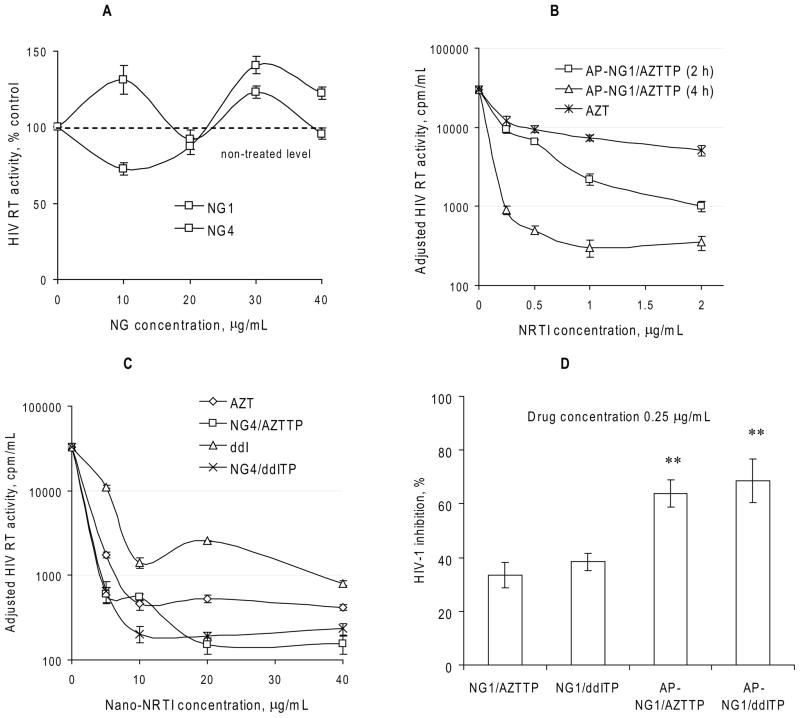
Figure 4.
Antiviral activity of Nano-NRTIs in HIV-1 infected MDMs. (A) HIV RT activity measured in cultural media on Day 5 after infection of MDMs pre-incubated for 2 h with nanogels without drug at concentrations 10–40 μg/mL (means ± SEM, n = 5). Activity in non-treated infected cells was taken for 100%. (B) Dose-dependent antiviral effect of AP-NG1/AZTTP pre-incubated with MDMs for 2 or 4 h at 37°C (means ± SEM, n = 5). HIV RT activity was adjusted by normalization using cytotoxicity data (MTT assay). (C) Antiviral effect-dose curves for Nano-NRTIs (for example, NG4/AZTTP and NG4/ddITP) and corresponding NRTIs (AZT and ddI) pre-incubated with MDMs for 2 h at 37°C (means ± SEM, n = 5). These curves were used for calculation of EC90 values presented in Table 2. (D) HIV-1 inhibition by non-vectorized and vectorized Nano-NRTIs. HIV RT activity was compared in MDMs after the pre-incubation for 4 h at 37°C with AZTTP and ddITP-loaded nanogels containing equal amount of drugs (0.25 μg/mL of AZT or ddI) followed by HIV-1 infection. Data are shown as means ± SEM (n = 5); (**) P < 0.01 between the corresponding non-vectorized and AP-vectorized Nano-NRTIs.