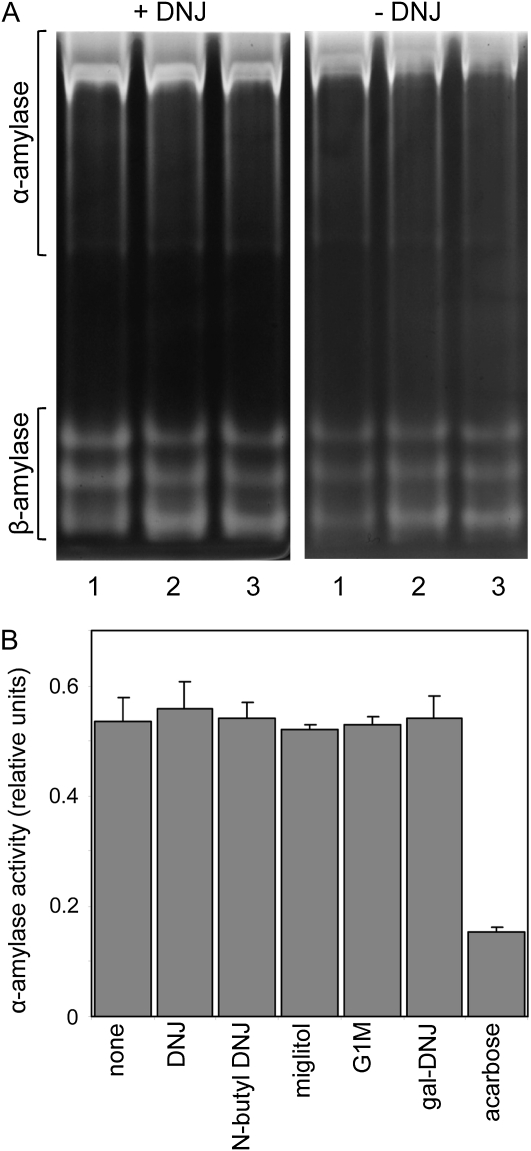
Figure 5.
Effects of inhibitors on starch-hydrolyzing activities in endosperm extracts. A, Extracts of seedlings 3 d after imbibition were subjected to electrophoresis on native, 7.5% acrylamide gels containing 0.1% (w/v) amylopectin. After incubation for 15 min at room temperature, gels were stained with iodine solution and washed to reveal bands attributable to starch-hydrolyzing enzymes. The left gel and the incubation medium contained 500 μm DNJ; the right gel and incubation medium contained no DNJ. Lane 1, No DNJ present during seedling growth or extraction; lane 2, 500 μm DNJ present during seedling growth but not during extraction; lane 3, 500 μm DNJ present during seedling growth and extraction. The two gels were run at the same time and photographed identically. Positions of bands attributable to α- and β-amylases are indicated. Attributions were confirmed by transfer of proteins after electrophoresis to gels containing β-limit dextrins. Bands attributed to α-amylase were visible after incubation, but bands attributed to β-amylase activity were not (data not shown). B, An extract of seedlings 4 d after germination was assayed for α-amylase activity in the presence or absence (none) of 500 μm inhibitor. Values are means of measurements on three technical replicates. Error bars represent se.