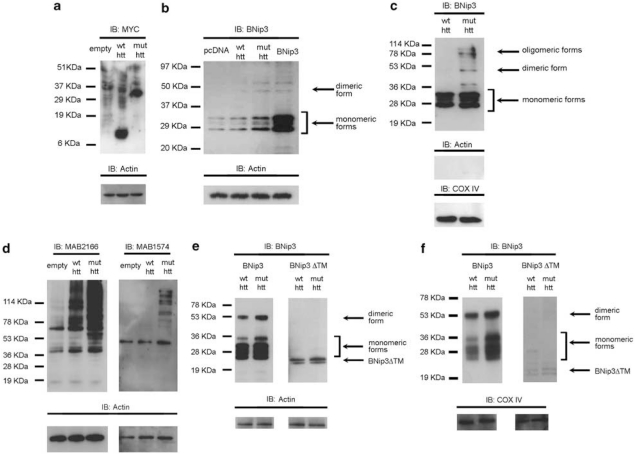
Figure 3.
Analysis of BNip3 levels in cell culture models expressing mutant htt. (a) WB analysis of whole-protein extracts from SHSY5Y cells transiently transfected with pcDNA4 (lane 1), wild-type htt exon1-9CAG (lane 2) or mutated htt exon1-60CAG (lane 3) and harvested 72 h after transfection. The WB was probed with anti-Myc antibody (both wild-type and mutant htt exon-1 were tagged C-terminally with Myc epitopes). (b) WB analysis of SHSY5Y cells transiently transfected with empty vector (lane 1), wild-type htt exon1-9CAG (lane 2) or mutant htt exon1-60CAG (lane 3). Cells were harvested 72 h after transfection. The signals corresponding to monomeric and dimeric BNip3 (Clone ANa40) were higher in cells expressing mutant htt than in those expressing wild-type htt. Results are representative of three independent experiments. To verify the specificity of the signal, SHSY5Y cells were transfected with a plasmid encoding BNip3 (lane 4). Results are representative of three independent experiments. (c) Equal amounts of protein from alkali-treated mitochondrial fractions of SHSY5Y cells expressing wild-type htt exon1-9CAG (lane 1) or mutant htt exon1-60CAG (lane 2) were analyzed by WB and probed with anti-BNip3 antibody (Clone ANa40). To verify equal protein loading, membranes were stripped and reprobed with anti-actin and anti-COX IV antibodies. Results are representative of three independent experiments. (d) WB analysis of HEK293T cells transiently transfected with empty pcMV6NEO (lane 1), wild-type full-length htt-17CAG (lane 2) or mutant full-length htt-47CAG (lane 3). Blots were probed with anti-htt (MAB2166) and anti-poly-glutamine (MAB1574) antibodies. (e) WB analysis of HEK293T cells transiently cotransfected with wild-type full-length htt-17CAG and BNip3 (left panel, lane 1); mutant full-length htt-47CAG and BNip3 (left panel, lane 2); wild-type full-length htt-17CAG and BNip3ΔTM (right panel, lane 3); or mutant full-length htt-47CAG and BNip3ΔTM (right panel, lane 4). The signals corresponding to monomeric and dimeric BNip3 were higher in cells expressing mutant htt than in those expressing wild-type htt. Results are representative of three independent experiments. (f) Equal amounts of protein from alkali-treated mitochondrial fractions were analyzed by WB and probed with anti-BNip3 antibody (Clone ANa40). HEK293T cells were transiently transfected with wild-type full-length htt-17CAG and BNip3 (left panel, lane 1); mutated full-length htt-47CAG and BNip3 (left panel lane 2); wild-type full-length htt-17CAG and BNip3ΔTM (right panel, lane 1); or mutant full-length htt-47CAG and BNip3ΔTM (right panel, lane 2). To verify equal protein loading, blots were stripped and reprobed with anti-COX IV antibodies. Results are representative of three independent experiments