Abstract
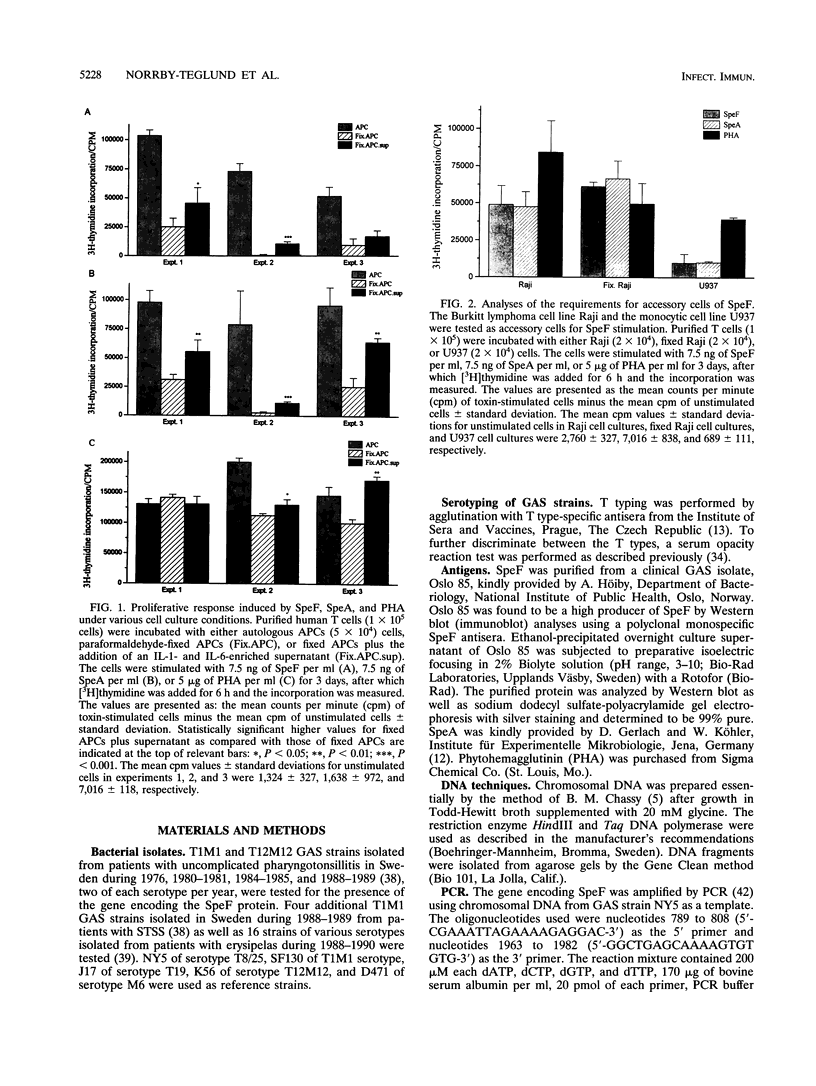
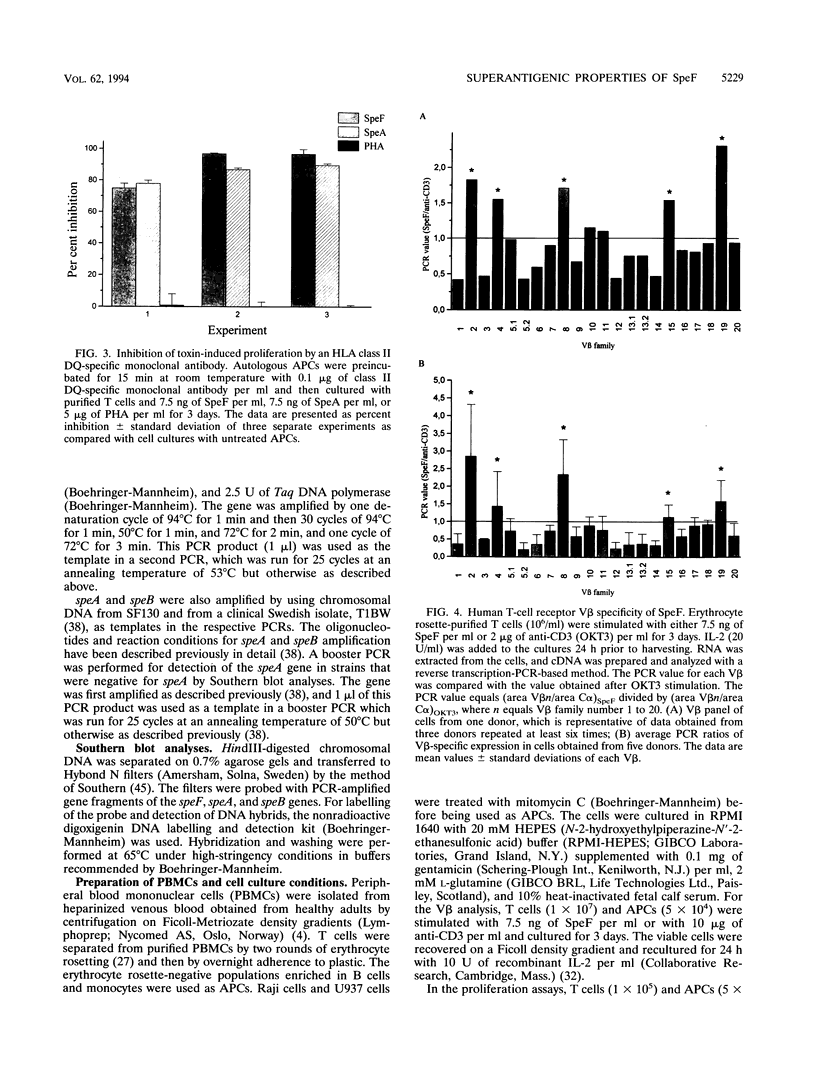
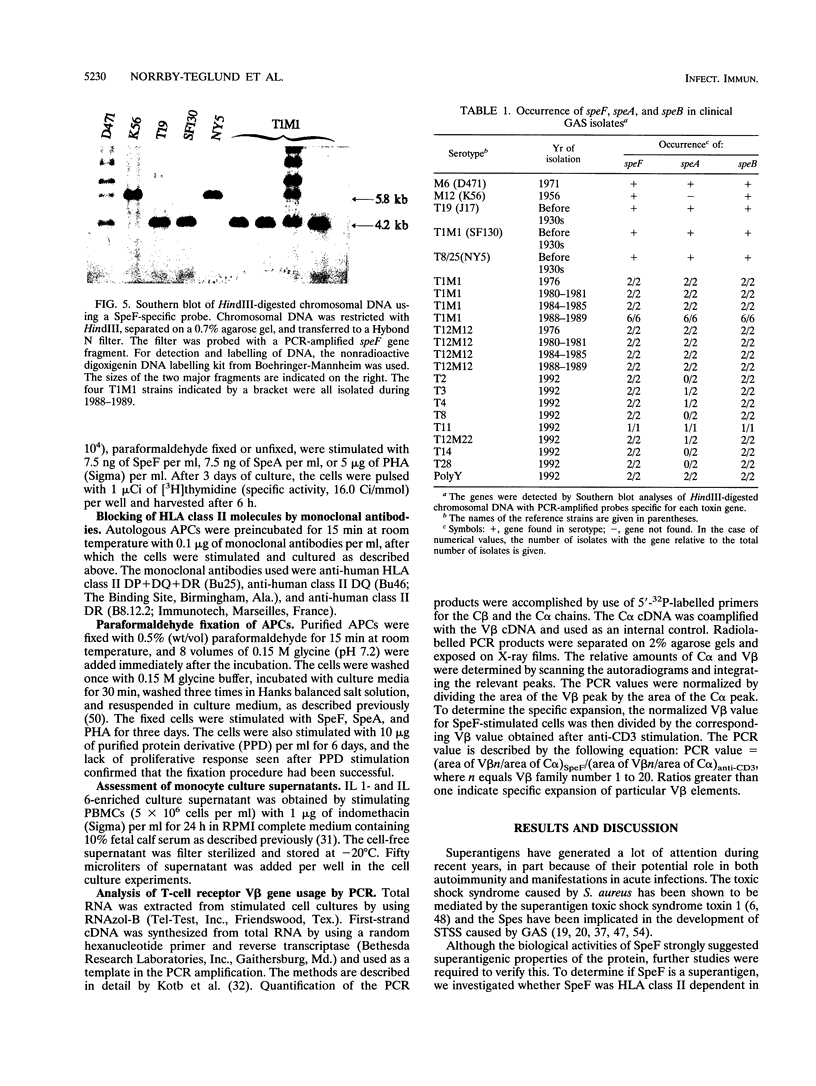
Streptococcal pyrogenic exotoxin F (SpeF), previously referred to as mitogenic factor, is a newly described potent mitogen produced by group A streptococci. To investigate whether this protein belongs to the family of microbial superantigens, we analyzed the cellular and molecular requirements for its presentation to T cells and compared it with the known streptococcal superantigen pyrogenic exotoxin A (SpeA) and the nonspecific polyclonal T-cell mitogen phytohemagglutinin (PHA). SpeF and SpeA were efficiently presented by autologous antigen-presenting cells (APCs) and an allogeneic B lymphoma cell line, Raji. In contrast, the monocytic cell line U937, which does not express major histocompatibility complex (MHC) class II molecules, failed to present SpeF as well as SpeA but supported the response to PHA. Thus, the presentation of SpeF by APCs was class II dependent but not MHC restricted. The requirement for HLA class II was further supported by the ability of anti-HLA-DQ monoclonal antibody to block the SpeF-induced proliferative response by 75 to 100%. Paraformaldehyde (PFA) fixation of autologous APCs resulted in an impaired ability of SpeF and SpeA to induce optimal T-cell proliferation. In contrast, fixation of Raji cells did not affect the induced proliferation. The stimulatory effect of PHA remained unaffected by both the use of PFA-fixed APCs and the addition of the HLA class II-specific monoclonal antibodies. The addition of a supernatant enriched in interleukin 1 and interleukin 6 to fixed autologous APCs resulted in an increased SpeF-induced response; thus, the impairment was not due to a requirement for processing, but, rather, costimulatory factors produced by metabolically active APCs were needed. SpeF was found to preferentially activate T cells bearing V beta 2, 4, 8, 15, and 19, as determined by quantitative PCR. The data presented clearly show that SpeF is a superantigen. We also studied the prevalence of the speF gene in clinical isolates by Southern blot analyses, and the gene could be detected in 42 group A streptococcal strains, which represented 14 serotypes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Nagy S., Björk L., Abrams J., Holm S., Andersson U. Bacterial toxin-induced cytokine production studied at the single-cell level. Immunol Rev. 1992 Jun;127:69–96. doi: 10.1111/j.1600-065x.1992.tb01409.x. [DOI] [PubMed] [Google Scholar]
- Arnold A., Lipkowitz S., Suthanthiran M., Novogrodsky A., Stenzel K. H. Human B lymphoblastoid cell lines provide an interleukin 1-like signal for mitogen-treated T lymphocytes via direct cell contact. J Immunol. 1985 Jun;134(6):3876–3881. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Choi Y., Lafferty J. A., Clements J. R., Todd J. K., Gelfand E. W., Kappler J., Marrack P., Kotzin B. L. Selective expansion of T cells expressing V beta 2 in toxic shock syndrome. J Exp Med. 1990 Sep 1;172(3):981–984. doi: 10.1084/jem.172.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaki Y., Fukui Y., Sudo T., Yamamoto K., Inamitsu T., Nishimura Y., Hirokawa K., Kimura A., Sasazuki T. Role of human major histocompatibility complex DQ molecules in superantigenicity of streptococcus-derived protein. Infect Immun. 1994 Apr;62(4):1228–1235. doi: 10.1128/iai.62.4.1228-1235.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fast D. J., Schlievert P. M., Nelson R. D. Toxic shock syndrome-associated staphylococcal and streptococcal pyrogenic toxins are potent inducers of tumor necrosis factor production. Infect Immun. 1989 Jan;57(1):291–294. doi: 10.1128/iai.57.1.291-294.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Gerardy-Schahn R., Metzroth B., Carrel S., Gerlach D., Köhler W. An evolutionary conserved mechanism of T cell activation by microbial toxins. Evidence for different affinities of T cell receptor-toxin interaction. J Immunol. 1991 Jan 1;146(1):11–17. [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H., Conradt P. T lymphocyte activation by staphylococcal enterotoxins: role of class II molecules and T cell surface structures. Cell Immunol. 1989 Apr 15;120(1):92–101. doi: 10.1016/0008-8749(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Fraser J. D. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature. 1989 May 18;339(6221):221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- Gerlach D., Knöll H., Köhler W. Isolierung und Charakterisierung von erythrogenen Toxinen. I. Untersuchung des von Streptococcus pyogenes, Stamm NY-5 gebildeten Toxins A. Zentralbl Bakteriol A. 1980;247(2):177–191. [PubMed] [Google Scholar]
- Goshorn S. C., Schlievert P. M. Bacteriophage association of streptococcal pyrogenic exotoxin type C. J Bacteriol. 1989 Jun;171(6):3068–3073. doi: 10.1128/jb.171.6.3068-3073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshorn S. C., Schlievert P. M. Nucleotide sequence of streptococcal pyrogenic exotoxin type C. Infect Immun. 1988 Sep;56(9):2518–2520. doi: 10.1128/iai.56.9.2518-2520.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett S. P., Stevens D. L. Streptococcal toxic shock syndrome: synthesis of tumor necrosis factor and interleukin-1 by monocytes stimulated with pyrogenic exotoxin A and streptolysin O. J Infect Dis. 1992 May;165(5):879–885. doi: 10.1093/infdis/165.5.879. [DOI] [PubMed] [Google Scholar]
- Hackett S. P., Stevens D. L. Superantigens associated with staphylococcal and streptococcal toxic shock syndrome are potent inducers of tumor necrosis factor-beta synthesis. J Infect Dis. 1993 Jul;168(1):232–235. doi: 10.1093/infdis/168.1.232. [DOI] [PubMed] [Google Scholar]
- Hauser A. R., Schlievert P. M. Nucleotide sequence of the streptococcal pyrogenic exotoxin type B gene and relationship between the toxin and the streptococcal proteinase precursor. J Bacteriol. 1990 Aug;172(8):4536–4542. doi: 10.1128/jb.172.8.4536-4542.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser A. R., Stevens D. L., Kaplan E. L., Schlievert P. M. Molecular analysis of pyrogenic exotoxins from Streptococcus pyogenes isolates associated with toxic shock-like syndrome. J Clin Microbiol. 1991 Aug;29(8):1562–1567. doi: 10.1128/jcm.29.8.1562-1567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. E., Norrby A., Bergholm A. M., Norgren M. Aspects of pathogenesis of serious group A streptococcal infections in Sweden, 1988-1989. J Infect Dis. 1992 Jul;166(1):31–37. doi: 10.1093/infdis/166.1.31. [DOI] [PubMed] [Google Scholar]
- Imanishi K., Igarashi H., Uchiyama T. Relative abilities of distinct isotypes of human major histocompatibility complex class II molecules to bind streptococcal pyrogenic exotoxin types A and B. Infect Immun. 1992 Dec;60(12):5025–5029. doi: 10.1128/iai.60.12.5025-5029.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki M., Igarashi H., Hinuma Y., Yutsudo T. Cloning, characterization and overexpression of a Streptococcus pyogenes gene encoding a new type of mitogenic factor. FEBS Lett. 1993 Sep 27;331(1-2):187–192. doi: 10.1016/0014-5793(93)80323-m. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Yagi J., Conrad P. J., Katz M. E., Jones B., Vroegop S., Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989 Feb;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Johnson L. P., Schlievert P. M. Group A streptococcal phage T12 carries the structural gene for pyrogenic exotoxin type A. Mol Gen Genet. 1984;194(1-2):52–56. doi: 10.1007/BF00383496. [DOI] [PubMed] [Google Scholar]
- Johnson L. P., Tomai M. A., Schlievert P. M. Bacteriophage involvement in group A streptococcal pyrogenic exotoxin A production. J Bacteriol. 1986 May;166(2):623–627. doi: 10.1128/jb.166.2.623-627.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupin C., Anderson S., Damais C., Alouf J. E., Parant M. Toxic shock syndrome toxin 1 as an inducer of human tumor necrosis factors and gamma interferon. J Exp Med. 1988 Mar 1;167(3):752–761. doi: 10.1084/jem.167.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Kapur V., Majesky M. W., Li L. L., Black R. A., Musser J. M. Cleavage of interleukin 1 beta (IL-1 beta) precursor to produce active IL-1 beta by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7676–7680. doi: 10.1073/pnas.90.16.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotb M., Majumdar G., Tomai M., Beachey E. H. Accessory cell-independent stimulation of human T cells by streptococcal M protein superantigen. J Immunol. 1990 Sep 1;145(5):1332–1336. [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Mollick J. A., Cook R. G., Rich R. R. Class II MHC molecules are specific receptors for staphylococcus enterotoxin A. Science. 1989 May 19;244(4906):817–820. doi: 10.1126/science.2658055. [DOI] [PubMed] [Google Scholar]
- Mollick J. A., Miller G. G., Musser J. M., Cook R. G., Grossman D., Rich R. R. A novel superantigen isolated from pathogenic strains of Streptococcus pyogenes with aminoterminal homology to staphylococcal enterotoxins B and C. J Clin Invest. 1993 Aug;92(2):710–719. doi: 10.1172/JCI116641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser J. M., Hauser A. R., Kim M. H., Schlievert P. M., Nelson K., Selander R. K. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2668–2672. doi: 10.1073/pnas.88.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Norrby A., Holm S. E. Genetic diversity in T1M1 group A streptococci in relation to clinical outcome of infection. J Infect Dis. 1992 Nov;166(5):1014–1020. doi: 10.1093/infdis/166.5.1014. [DOI] [PubMed] [Google Scholar]
- Norrby-Teglund A., Norgren M., Holm S. E., Andersson U., Andersson J. Similar cytokine induction profiles of a novel streptococcal exotoxin, MF, and pyrogenic exotoxins A and B. Infect Immun. 1994 Sep;62(9):3731–3738. doi: 10.1128/iai.62.9.3731-3738.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby-Teglund A., Pauksens K., Holm S. E., Norgren M. Relation between low capacity of human sera to inhibit streptococcal mitogens and serious manifestation of disease. J Infect Dis. 1994 Sep;170(3):585–591. doi: 10.1093/infdis/170.3.585. [DOI] [PubMed] [Google Scholar]
- Norrby A., Eriksson B., Norgren M., Rönström C. J., Sjöblom A. C., Karkkonen K., Holm S. E. Virulence properties of erysipelas-associated group A streptococci. Eur J Clin Microbiol Infect Dis. 1992 Dec;11(12):1136–1143. doi: 10.1007/BF01961132. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schlievert P. M. Role of superantigens in human disease. J Infect Dis. 1993 May;167(5):997–1002. doi: 10.1093/infdis/167.5.997. [DOI] [PubMed] [Google Scholar]
- Scholl P., Diez A., Mourad W., Parsonnet J., Geha R. S., Chatila T. Toxic shock syndrome toxin 1 binds to major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4210–4214. doi: 10.1073/pnas.86.11.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stevens D. L., Tanner M. H., Winship J., Swarts R., Ries K. M., Schlievert P. M., Kaplan E. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med. 1989 Jul 6;321(1):1–7. doi: 10.1056/NEJM198907063210101. [DOI] [PubMed] [Google Scholar]
- Todd J. K. Toxic shock syndrome. Clin Microbiol Rev. 1988 Oct;1(4):432–446. doi: 10.1128/cmr.1.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomai M. A., Beachey E. H., Majumdar G., Kotb M. Metabolically active antigen presenting cells are required for human T cell proliferation in response to the superantigen streptococcal M protein. FEMS Microbiol Immunol. 1992 Feb;4(3):155–164. doi: 10.1111/j.1574-6968.1992.tb04982.x. [DOI] [PubMed] [Google Scholar]
- Tomai M., Kotb M., Majumdar G., Beachey E. H. Superantigenicity of streptococcal M protein. J Exp Med. 1990 Jul 1;172(1):359–362. doi: 10.1084/jem.172.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Imanishi K., Saito S., Araake M., Yan X. J., Fujikawa H., Igarashi H., Kato H., Obata F., Kashiwagi N. Activation of human T cells by toxic shock syndrome toxin-1: the toxin-binding structures expressed on human lymphoid cells acting as accessory cells are HLA class II molecules. Eur J Immunol. 1989 Oct;19(10):1803–1809. doi: 10.1002/eji.1830191007. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Kamagata Y., Yan X. J., Kohno M., Yoshioka M., Fujikawa H., Igarashi H., Okubo M., Awano F., Saito-Taki T. Study of the biological activities of toxic shock syndrome toxin-1: II. Induction of the proliferative response and the interleukin 2 production by T cells from human peripheral blood mononuclear cells stimulated with the toxin. Clin Exp Immunol. 1987 Jun;68(3):638–647. [PMC free article] [PubMed] [Google Scholar]
- WATSON D. W. Host-parasite factors in group A streptococcal infections. Pyrogenic and other effects of immunologic distinct exotoxins related to scarlet fever toxins. J Exp Med. 1960 Feb 1;111:255–284. doi: 10.1084/jem.111.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]