Abstract
Characterization of materials developed for medical usage frequently includes studies in which the materials are inoculated with bacteria in order to assess bacterial colonization and biofilm formation. Observed differences in bacterial growth are typically considered to be due to the material or the incubation conditions. To our knowledge, the method used to prepare the materials has generally not been considered with regard to its influence on bacterial colonization. The objective of this study was to determine the effects that various preparation methods exert on bacterial colonization of polymer disks. Polymer disks of the same dimethacrylate composition were photopolymerized: (1) between untreated glass slides, (2) between polyester release film, (3) between glass slides treated with an alkyl silane, (4) between glass slides treated with a perfluorinated silane, or (5) with one free surface in an argon-purged chamber. Surface chemistry was quantified using X-ray photoelectron spectroscopy, hydrophobicity was assessed by water contact angle, and topography was characterized using atomic force microscopy. The disks were inoculated with Streptococcus mutans for 4 h, fixed, and visualized using confocal laser scanning microscopy. Differences among all groups were found with regard to surface chemistry, hydrophobicity, topography, and bacteria morphology, density, and coverage, indicating that the method of sample preparation strongly affects both the surface properties and the initial bacterial colonization. Polymerization on untreated slides was selected as the preferred method of preparation due to minimal material transfer to the polymer and consistent, reproducible bacterial colonization.
Introduction
The study of biofilm growth on materials is attracting interest in a number of fields ranging from medicine to infrastructure. Biofilms can be beneficial, for example in wastewater treatment and soil remediation, but they can also be problematic in systems as diverse as marine biofouling or infected medical devices, and they are the primary cause of tooth decay (caries).1 Biofilms are dynamic, alternating between periods of growth and reduction,2 and biofilm formation occurs in three basic stages, beginning with attachment of bacteria to a surface. In the second stage, the bacteria proliferate and aggregate to form colonies, which then secrete extracellular polymeric substances (EPS) and eventually develop into a mature biofilm. During the final stage of biofilm growth bacteria are released into the planktonic phase,1-3 possibly due to a variety of reasons including host response, sloughing, or mechanical interaction. Many factors influence the attachment and longer-term adhesion of bacteria to surfaces4, including direct chemical bonding or electrostatic interactions between the bacteria and the substrate, environment (including local chemistry and flow conditions), and substrate properties such as surface hydrophobicity and topography.
It is important to test the affinity of pathogenic bacteria for new and existing biomaterials. Adherent bacteria have been observed on permanent medical devices such as heart valve replacements and orthopedic implants, on dental restorations, and on temporary devices including urinary catheters and contact lenses.5 Infections caused by these bacteria lead to additional health problems for the host and frequently to device failure. In the case of polymeric dental restoratives, slabs or disks of the material to be tested are typically used as a surface on which the bacteria are inoculated. The preparation of such samples for bacterial adhesion experiments has been carried out using a number of methods. For example, the material is often placed in a silicone,6, 7 aluminum,8 or other metallic9 mold, then photopolymerized directly against glass,10 or films of polyester11 or celluloid.12, 13 In addition, removal of polymerized materials from glass can be facilitated by altering the surface potential of the glass through deposition of a self-assembled monolayer (SAM) of an organosilane.14 The polymerized samples are sometimes polished, which further alters the surface functionality and morphology. To our knowledge, no studies have explored the effects that the method of sample preparation has upon bacterial colonization, the first step in biofilm formation. In this paper, we investigate the effects of sample preparation on surface chemistry, hydrophobicity, and topography of polymer disks and on the subsequent bacterial colonization.
Materials and Methods1
Substrate preparation
Glass slides (75 mm × 50 mm × 1 mm) and cover slips (18 mm × 18 mm) were cleaned with acetone prior to surface treatment. Slides were used for preparing polymer disks, and cover slips were used for contact angle measurements. Substrates were prepared according to the groups listed in Table 1, including untreated (Unt) glass slides and biaxially-oriented polyethylene terephthalate (PET) film cleaned with acetone, then placed on clean glass slides. To prepare n-octadecyltrimethoxysilane (OTMS; Gelest, Inc; Morristown, PA) surface treated substrates, 0.1 g OTMS was dissolved in 10 g acetone and 2 drops of acetic acid. Clean glass slides were dipped in the solution; excess solvent was evaporated in air and the slides were heat-treated in a 60 °C oven for 2 h. For (tridecafluoro-1,1,2,2-tetrahydrooctyl)dimethylchlorosilane (PFCS; Gelest, Inc; Morristown, PA)) surface treated substrates, a self-assembled monolayer (SAM) of PFCS was vacuum-deposited onto clean slides at room temperature, followed by an ethanol wash.
Table 1.
Opposing substrates used to prepare polymer disks
| Group | Opposing substrate |
|---|---|
| Unt | Untreated glass |
| Arg | No opposing surface; argon-purged chamber |
| PET | Biaxially-oriented polyethylene terephthlate |
| OTMS | Glass treated with n-octadecyltrimethoxysilane |
| PFCS | Glass vapor-deposited with (tridecafluoro-1,1,2,2- tetrahydrooctyl)dimethylchlorosilane |
Polymer disk preparation
A mixture of bisphenol A diglycidylether methacrylate/triethyleneglycol-dimethacrylate (BisGMA:TEGDMA, 50:50 mass ratio; Esstech, Essington, PA) activated with camphorquinone (0.2 % mass fraction; Aldrich; Milwaukee, WI) and ethyl-4-(dimethylamino)benzoate (0.8 % mass fraction; Aldrich; Milwaukee, WI) was used to prepare all disks. The prepared glass slides were cleaned with acetone, polytetrafluoroethylene (Teflon) spacers were placed on the slides to control disk thickness, and drops of the resin were placed on the slide, covered with another slide from the same treatment group (except in the case of Arg disks, which were prepared in an Argon-purged chamber), clamped together and placed in a Triad 2000 Visible Light Cure System (Dentsply; York, PA) for 1 min per side for photopolymerization. Argon-purged disks (Arg) were prepared by placing the resin mixture in Teflon molds, leaving the top surface free. The molds were placed in a chamber, and the exposed surface was purged with argon for 10 min and photopolymerized for 2 min. All disks (diameter ≈ 6 mm, thickness ≈ 1.6 mm) were sterilized in 70 % (volume fraction) ethanol for 20 min, rinsed twice with phosphate-buffered saline (PBS), and stored in PBS overnight.
X-ray photoelectron spectroscopy (XPS)
XPS was performed on the polymerized disks that had been soaked in ethanol in the same manner as the disks used for bacterial colonization. Spectra were obtained on a Kratos AXIS Ultra DLD spectrometer (Kratos Analytical, Manchester, UK) with a monochromatic Al X-ray source (1486.7 eV) operating at 140 W under 1.0 × 10−7 Pa vacuum. Measurements were performed in hybrid mode using electrostatic and magnetic lenses, and the take-off angle was 0° (angle between the sample surface normal and the electron optical axis of the spectrometer), which yields a maximum sampling depth of approximately 8 nm.15 Atomic concentrations were calculated from survey spectra, collected over a binding-energy range from 1100 eV to 0 eV using a pass energy of 160 eV, energy resolution of 0.2 eV, and a 500 ms dwell time. A flood gun was used for charge neutralization, and all spectra were shifted with respect to the C 1s peak at 285 eV. One spectrum from each of two samples at each preparation condition was obtained. The standard uncertainty of the XPS measurements is 2 %.
Contact angle measurements
Water contact angle measurements were taken on a sessile drop of deionized water dispensed from a blunt needle using an NRL Contact Angle Goniometer, Model 100-00 (Ramé-Hart, Inc, Mountain Lakes, NJ). All measurements were taken at room temperature and ambient atmosphere. At least four measurements were taken on each of four samples for disks and treated glass cover slips.
Atomic Force Microscopy (AFM)
AFM images were recorded using a Digital Instruments Dimension 3100 scanning force microscope in tapping mode equipped with Point Probe Plus Non-Contact /Tapping Mode - Long Cantilever (PPP-NCL) cantilevers from Asylum Research (point probe plus, non-contact, low resonance, spring constant ranging between 21 N/m and 98 N/m and a resonant frequency of 146 kHz to 236 kHz). For each sample, at least three different positions were scanned and representative height and phase images were recorded. The images were analyzed using Nanoscope 5.12r2 software. Root mean square roughness (RRMS) was calculated from the height images. The standard uncertainty of the AFM height measurements is 2 %.
Bacteria culture and inoculation
Streptococcus mutans (S. mutans, Clarke UA159, American Type Culture Collection) were cultured at 37 °C in Bacto Brain-Heart Infusion broth. In preparation for inoculating the disks, bacteria were centrifuged at 418.9 rad/s for 10 min at 4 °C. The pellet was resuspended in PBS, centrifuged again, and resuspended in PBS containing 100 mg/L MgCl2·6H2O and 100 mg/L CaCl2 for a final optical density of 0.06 at λ = 600 nm. Sterilized polymer disks were placed in 12-well or 24-well tissue culture plates and inoculated with 2 mL or 1 mL of the bacteria suspension/well, respectively. As a negative control, sterilized, untreated glass cover slips were placed in 6-well tissue culture plates and inoculated with 5 mL of bacteria suspension. These volumes were calculated to provide the same concentration of bacteria per unit area for all well plates. After incubating for 4 h at 37 °C and 5 % CO2, samples were gently washed by three exchanges of half the solution with fresh PBS and then fixed for 30 min using 3.7 % (mass fraction) formaldehyde. Samples were not passed through the air-liquid interface until after fixation. Bacteria were then incubated for 30 min with 1 μmol/L SYTOX Green in PBS to stain the nucleic acids. Samples were stored hydrated in the dark at 4 °C.
Confocal laser scanning microscopy (CLSM) and image analysis
Samples were immersed in PBS and imaged on a Zeiss LSM 510 confocal laser scanning microscope using an Achroplan IR 40x/0.80 W water immersion objective. Z-stacks (230.3 μm × 230.3 μm × 1 μm) were collected at five random locations on each of three disks and included the entire thickness of the bacterial layer at each location. Three-dimensional (3D) projections of stacks were prepared using the Zeiss software package and converted to tagged image file format. A macro was written in ImagePro Plus software to quantify bacteria colonization on the surface. The macro evaluated the bacteria as objects, where an object is defined as a single fluorescent entity representing either a single bacterium or a microcolony of multiple bacteria. First, the area of each individual object was measured. Then, the object density was determined as the number of objects per unit area, and the fraction of the surface covered by bacteria was calculated for each image as the area covered by bacteria divided by the total image area.
Statistical analysis
Contact angle data were analyzed using One-Way Analysis of Variance (ANOVA) with a Bonferroni multiple comparison test at a significance level of 0.05. Individual object area data were log-transformed to approximate normality and analyzed using One-Way ANOVA with Tukey multiple comparisons performed at a 95 % confidence. The nonparametric Kruskal-Wallis test at 95 % confidence was used to compare populations for object density and fraction covered, as the data were not found to conform to any simple established statistical distribution.
Results and Discussion
Surface chemistry and topography are known to affect bacterial colonization of substrates, which in turn affects biofilm formation.16 In this study, the effects of sample preparation on surface chemistry, hydrophobicity, and roughness, and on the resulting bacterial morphology were evaluated. Subtle differences in surface properties were detected and found to result in significant changes in the initial bacterial colonization. Based on the results, a preferred sample preparation method was identified.
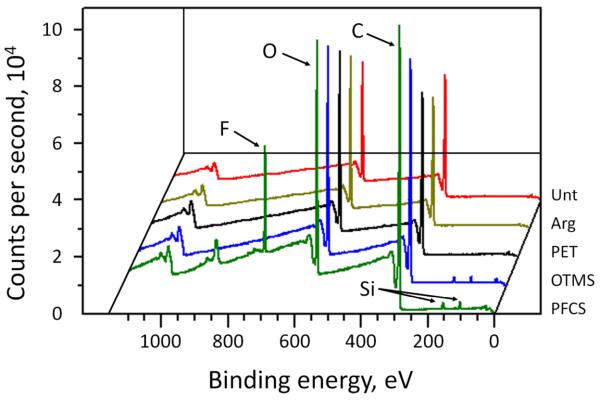
The chemical compositions of the polymer disks, as measured via XPS, depended upon the preparation method, with the chemical analysis supporting a transfer of material from the opposing substrate to the dimethacrylate disks. XPS results (Figure 1) revealed the presence of carbon (285 eV) and oxygen (530 eV) along with a small amount of silicon (100 eV and 150 eV) and in some cases fluorine (703 eV). Silicon was detected on the surfaces of all disks but was not a part of the material but rather a trace contaminant. Arg disks had the smallest amount of silicon as they did not come in direct contact with an opposing glass substrate, and therefore serve as the baseline from which silicon transfer to other disks can be evaluated (Table 2). A modest increase in silicon was observed for Unt disks, and larger increases in silicon were observed for PET, OTMS and PFCS disks. Nitrogen was detected on PET disks only, suggesting a transfer of material from the PET film to the polymer disks, including the transfer of possible nitrogen-containing contaminants present on the PET film. In addition, fluorine was detected on disks prepared on glass treated with a perfluorinated silane, supporting the transfer of the silane coating to the disks. Boron was found on disks prepared on untreated glass slides, consistent with material transfer from borosilicate glass. XPS was conducted on disks after ethanol sterilization and PBS soaking, indicating these processes do not completely remove the transferred material and that the bacteria were exposed to surfaces with these functionalities. It is clear, therefore, that the opposing substrate affects the surface chemistry of the polymer disks via material transfer from the substrate to the polymer. Since BisGMA and TEGDMA are often used as adhesives,17 it is not surprising that material transfer from the opposing substrate to the adhesive polymers was evident.
Figure 1.
XPS analysis of BisGMA:TEGDMA disks prepared on different substrates.
Table 2.
XPS analysis of polymer disks (% Composition)*
| Group | C | O | Si | F |
|---|---|---|---|---|
| Unt | 77.02 ± 0.04 | 22.00 ± 0.33 | 0.30 ± 0.03 | -- |
| Arg | 76.62 ± 0.30 | 23.22 ± 0.17 | 0.16 ± 0.13 | -- |
| PET | 76.62 ± 0.28 | 20.78 ± 0.00 | 1.85 ± 0.71 | -- |
| OTMS | 78.32 ± 0.25 | 20.29 ± 0.04 | 1.40 ± 0.21 | -- |
| PFCS | 77.39 ± 1.84 | 20.85 ± 0.33 | 1.66 ± 1.55 | 0.12 ± 0.08 |
Unt disks also contained sulfur (0.19 ± 0.11), calcium (0.16 ± 0.16), and boron (0.33 ± 0.03). PET disks also contained nitrogen (0.75 ± 0.42). Measurements are reported as the average ± one standard deviation.
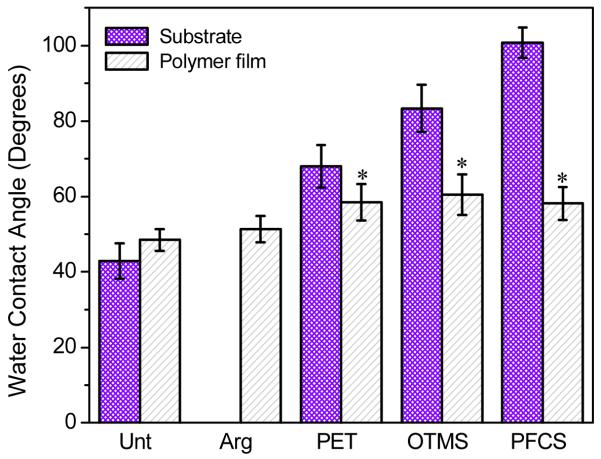
These variations in surface chemistry may account, in part, for the differences seen in the contact angles of the polymer disks. Figure 2 shows the water contact angles of the opposing substrates and the resulting dimethacrylate disks. Substrates of increasing hydrophobicity were used. The mean contact angles of the Unt disks and Arg disks (≈ 49 °) were statistically identical. An increase in water contact angle (≈ 57 °) was observed for disks prepared using PET, OTMS, and PFCS (p ≤ 0.05). These disks had statistically identical contact angles even though the contact angle of their opposing substrates was increasing, indicating a potential plateau for the contact angle for the disks. Overall, the surface hydrophobicity of dimethacrylate disks was affected by the preparation method, with polymers with greater surface contamination as confirmed via XPS (PET, OTMS, PFCS) having increased contact angles. In addition to material transfer, preferential change rearrangement during the photopolymerization process may also contribute to the changes in contact angle, with more hydrophobic opposing substrates resulting in a polymer surface rich in the hydrophobic domains of the monomers.
Figure 2.
Contact angle measurements of BisGMA:TEGDMA disks and the substrates on which they were prepared (Arg disks were prepared with no opposing substrate). * indicates a significant difference (p ≤ 0.05) for disks compared to the Unt disks. Bar heights are the averages (n ≥ 16), and error bars represent one standard uncertainty (1 σ).
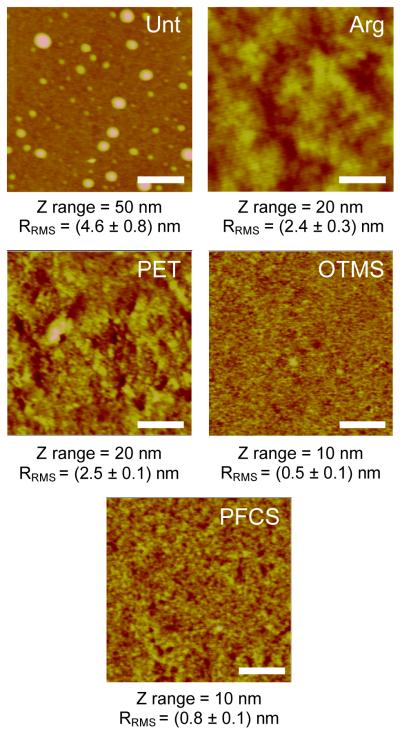
Examination of the dimethacrylate disks using AFM revealed striking differences in topography between Unt disks and those prepared using the other treatments (Figure 3). Nodules, ≈ 50 nm in height and ranging from ≈ 50 nm to ≈ 150 nm in diameter, were randomly distributed on the surface of the Unt disks. Phase images taken concurrently with the height images (Figure S1) showed that the nodules were composed of the same material as the underlying surface, indicating that they were not transferred particles of glass that had bonded to the disk and detached from the glass substrate. Disks prepared on the other substrates had a smaller height range and did not exhibit distinct features (Figure 3). Phase images of the polymer disks (Figure S1) were not indicative of substantial material transfer from the substrates on which they were prepared. Thus, the material transfer evident from the XPS results must be minimal. AFM images of Unt glass substrates and OTMS glass substrates (Figure S2A) reveal topographical disparities between the substrates, with the Unt glass showing nodules similar to those on the Unt polymer disks. It is possible that these particles are left on the glass during the manufacturing process and transferred to the polymer disks during sample fabrication. OTMS-treated glass surfaces are somewhat smoother, with undulating surface irregularities. Nodules are present in some regions, however. Phase images (Figure S2B) indicate areas with changing material composition on the OTMS-treated glass, suggestive of incomplete or irregular coverage of the glass with the silane. Irregular coverage on the glass may result in non-uniform transfer from the substrate to the polymer disks.
Figure 3.
AFM height images of the varying surface topographies for disks prepared on different substrates. Scale bars = 500 nm. RRMS is presented as the average and standard deviation of three samples.
RRMS, as calculated from AFM height images, varied with disk preparation but did not correlate with surface hydrophobicity. For example, the mean water contact angles for PET, OTMS, and PFCS disks were statistically identical, yet the RRMS for these three disks varied substantially (Figure 3). The RRMS for PET was closer to the value measured for the less hydrophobic Unt and Arg disks. Likewise, no clear correlative trends were evident between surface roughness and surface contamination (transferred from the substrate to the polymer) detected via XPS.
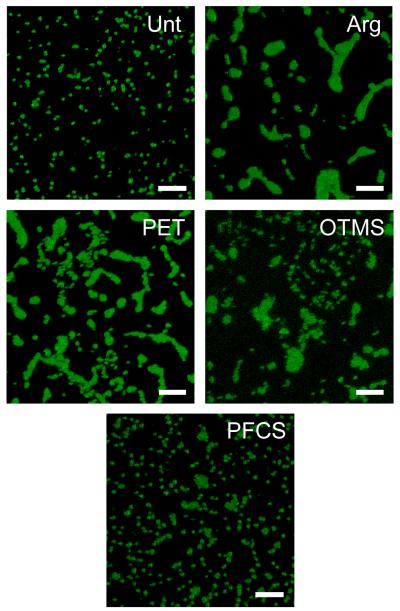
Bacterial adhesion, the first step in biofilm formation, is sensitive to a large number of factors including local chemistry, topography, and fluid flow.16 In order for meaningful conclusions to be drawn from bacterial adhesion experiments, bacterial behavior must be consistent throughout repeated experiments and within the same experiment. As Figure 4 shows, bacterial colonization differed markedly not only between, but also within some of the single treatments, with bacterial morphology varying widely for all but Unt disks. The bacteria were consistently individual on the Unt disks but aggregated irregularly into microcolonies on disks prepared using other treatments. Bacteria cultured on disks prepared in argon were primarily grouped into large, rounded and elongated microcolonies. The distribution of object areas was skewed towards these larger objects although some individual bacteria were still visible. Bacteria cultured on disks prepared using PET, OTMS, and PFCS substrates showed heterogeneous morphologies consisting of both individual bacteria and unevenly distributed microcolonies varying greatly in size and shape.
Figure 4.
CLSM images of S. mutans cultured on BisGMA:TEGDMA disks for 4 h. Images reveal variations in microcolony formation, depending on disk fabrication method. Scale bars = 10 μm.
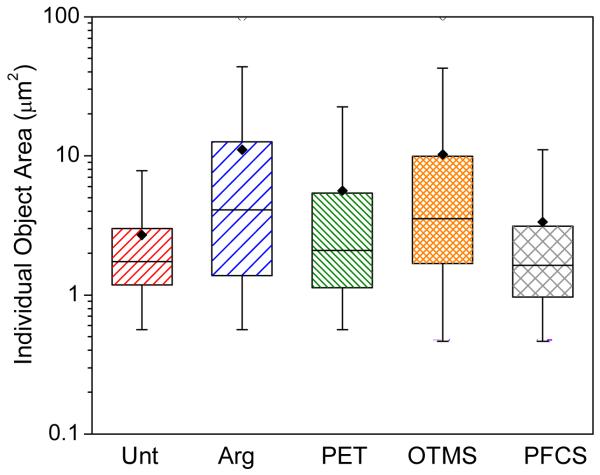
The effects exerted by the different surface treatments on bacterial colonization were validated quantitatively by image analysis. Figure 5 plots the areas of individual objects as a function of opposing substrate. Each object was either a single bacterium or a microcolony of bacteria. The spread in object area for Arg, PET, OTMS and PFCS was broader than that for Unt, with a greater propensity towards larger objects and larger mean object areas. The distribution of object areas was determined to be lognormal. Statistical analysis shows that the average log object area on each disk is significantly different from that on all other disks. Thus, the bacteria are sensing and responding to differences in the disk surfaces.
Figure 5.
Box plot of the areas of individual objects, as quantified from CLSM images of S. mutans on the disks. At least 9600 objects were measured per treatment. Each box represents the interquartile range. The inner line in each box represents the median for that sample type, while the mean is indicated by the black diamond.
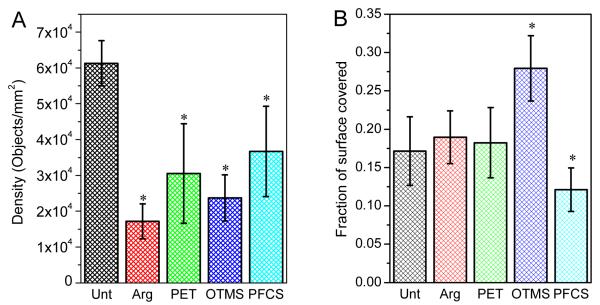
Object density (objects per area) and fractional coverage of the surface for the dimethacrylate disks reveal additional differences in the bacterial colonization (Figure 6). Unt disks had significantly more objects than all other disks, which would be expected since Unt disks has similar surface coverage to the other disks but a smaller average object area and standard deviation. However, PFCS disks, which had only slightly larger objects than Unt, had a significantly lower object density than Unt. As the fraction covered data indicate, PFCS disks had a lower fraction covered than all other disks (Figure 6B), so it follows that PFCS disks should have fewer objects than Unt due to the lower surface coverage. The presence of larger objects on the Arg, PET, and OTMS disks as compared to Unt disks (Figure 5) resulted in a lower object density compared to Unt (Figure 6A), even for OTMS which had a significantly larger fraction covered compared to the other disks. Thus, while the correlation between object area and object density is similar to the expected inverse relationship, no clear correlation exists between object area and fraction of surface covered or between fraction covered and object density.
Figure 6.
Object density (A) and fraction of surface covered (B) on disks inoculated with S. mutans. * indicates a significant difference (p ≤ 0.05) compared to Unt (Kruskal-Wallis test). Data represent the average values (n = 15). Error bars represent one standard uncertainty (1 σ).
Changes in the method of disk preparation resulted in multiple topographical and chemical effects that could not be separated and investigated independently, thus the bacterial response to the combined effects must be considered accordingly. In evaluating the data, few correlations were identified. Quantifiable properties of the bacterial colonization, including object area and fraction of surface covered, varied widely and did not correlate with one another. Object size and density, as well as surface coverage, showed no relation to surface hydrophobicity, unlike some previous studies that indicated an inverse correlation between surface free energy and bacterial adhesion18. Disks prepared on PET, OTMS, and PFCS substrates were more hydrophobic than those prepared on untreated glass slides, yet the morphology of the bacteria were visibly different among the treatments. The mean water contact angle of disks prepared in argon was statistically identical to that of disks prepared on untreated slides, yet bacteria cultured on Arg disks adhered to form the largest groups while bacteria on Unt disks remained individual. Although it has been shown that S. mutans show preferential adhesion to substrates with high surface free energy,19 our study had multiple surface properties changing on the polymer disks, making it difficult, if not impossible, to rule out the possibility of a correlation between hydrophobicity and bacterial colonization. Likewise, while there were significant differences in the contact angle, the absolute differences were less than 10° and may not be sufficient to change the bacterial colonization.
One probable cause for the disparities in bacterial colonization on disks prepared with PET, OTMS, and PFCS substrates, when compared with the relatively regular bacteria colonization on the Unt and Arg disks, is variable material transfer from the opposing substrate to the polymer disk. XPS analysis indicated nitrogen on the PET sample surface and fluorine contamination on PFCS disks, evidence of material transfer despite thorough cleaning of the PET film and PFCS-treated glass prior to sample preparation. Likewise, excess OTMS probably remained on the glass surface after treatment and washing.
When considering surface topography, studies have demonstrated that more oral bacteria adhere to and more dental plaque develops on rougher surfaces.20-22 Experimental evidence has shown that bacterial colonization is sensitive to both micro- (RRMS > 200 nm)23 and nano-scale (RRMS < 10 nm)24. Yet Unt and PFCS, which appeared to yield the most similar bacterial colonization morphologies (Figure 4), had RRMS measurements that differed far more than did Unt and Arg (Figure 3), on which the bacteria had very different object areas. Moreover, the size of the nodules was likely too small to be detected by the bacteria. These differences in surface roughness are small compared to the size of the bacteria and may be due to the slight material transfer to the polymer disks. Taken together, the material transfer and its effects on contact angle and surface roughness may be the driving force behind the disparities seen in bacterial colonization. Since the material is not controllable and is likely not uniform, the preparation method that results in the least material transfer should have the greatest reproducibility and consistency. In considering all the data, polymerizing disks using untreated glass slides (Unt) minimized material transfer to the polymers.
Chemical and surface analyses have shown that material surface properties depend upon the opposing substrate used during photopolymerization, and the resulting bacterial colonization is quantifiably different across the treatment groups. Under these experimental conditions it is not possible to isolate the chemical and topographical effects of a given treatment and therefore delineate the correlation or lack thereof between material properties and colonization. Nevertheless, the presence of these chemical and topographical differences and their effects indicates that the consequences of sample preparation method are not trivial. Furthermore, substantial variations in bacterial colonization were found within all sample groups except for Unt. Although the topography of Unt disks differed from that of the other groups, the consistency of the bacterial colonization within this group, along with the relatively small degree of material transfer, as shown via surface contamination measured by XPS, indicates that Unt disks can be expected to yield the most reproducible results in terms of both surface properties and initial bacterial colonization.
Conclusions
The method by which materials are prepared for bacterial colonization studies greatly influences the outcome. Until this time, preparation methods have not been considered to affect the morphology of bacteria cultured on a given material, yet here we demonstrate that even subtle differences in the surface properties can have very significant effects on the bacterial colonization, and must therefore be characterized and considered when evaluating a material’s antimicrobial capabilities. The technique by which a polymer disk is prepared affects its surface chemistry, hydrophobicity, and topography, and in turn, the bacterial colonization. For studies to be properly conducted, samples should be fabricated by methods that yield consistent products, and the resulting material properties should be characterized. When all evaluated properties were compared and considered, disks prepared on untreated glass slides yielded results with the greatest consistency and reproducibility.
Supplementary Material
Acknowledgements
This work is supported by Interagency Agreement Y1-DE-7005-01 between the National Institute of Dental and Craniofacial Research (NIDCR) and the National Institute of Standards and Technology (NIST).
Footnotes
Certain equipment, instruments or materials are identified in this paper in order to adequately specify the experimental details. Such identification does not imply recommendation by the National Institute of Standards and Technology nor does it imply the materials are necessarily the best available for the purpose.
Supporting Information Available: AFM phase images of dimethacrylate disks and AFM height and phase images of untreated and OTMS-treated glass. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Nadell CD, Xavier JB, Foster KR. FEMS Microbiol. Rev. 2009;33:206. doi: 10.1111/j.1574-6976.2008.00150.x. [DOI] [PubMed] [Google Scholar]
- (2).Xavier JB, Picioreanu C, van Loosdrecht MC. Biotechnol. Bioeng. 2005;91:651. doi: 10.1002/bit.20544. [DOI] [PubMed] [Google Scholar]
- (3).Sissons CH. Adv. Dent. Res. 1997;11:110. doi: 10.1177/08959374970110010201. [DOI] [PubMed] [Google Scholar]
- (4).Geoghegan M, Andrews JS, Biggs CA, Eboigbodin KE, Elliott DR, Rolfe S, Scholes J, Ojeda JJ, Romero-González ME, Edyvean RGJ. Faraday Discus. 2008;139:85. doi: 10.1039/b717046g. [DOI] [PubMed] [Google Scholar]
- (5).Bryers JD, Hendricks S. Ann. N. Y. Acad. Sci. 1997;831:127. [PubMed] [Google Scholar]
- (6).Senawongse P, Pongprueksa P. J. Esthet. Restor. Dent. 2007;19:265. doi: 10.1111/j.1708-8240.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- (7).Tanner J, Carlen A, Soderling E, Vallittu PK. J. Biomed. Mater. Res. B Appl. Biomater. 2003;66:391. doi: 10.1002/jbm.b.10012. [DOI] [PubMed] [Google Scholar]
- (8).Sideridou I, Tserki V, Papanastasiou G. Biomaterials. 2003;24:655. doi: 10.1016/s0142-9612(02)00380-0. [DOI] [PubMed] [Google Scholar]
- (9).Montanaro L, Campoccia D, Rizzi S, Donati ME, Breschi L, Prati C, Arciola CR. Biomaterials. 2004;25:4457. doi: 10.1016/j.biomaterials.2003.11.031. [DOI] [PubMed] [Google Scholar]
- (10).Gyo M, Nikaido T, Okada K, Yamauchi J, Tagami J, Matin K. Appl. Environ. Microbiol. 2008;74:1428. doi: 10.1128/AEM.02039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Satou J, Fukunaga A, Satou N, Shintani H, Okuda K. J. Dent. Res. 1988;67:588. doi: 10.1177/00220345880670031301. [DOI] [PubMed] [Google Scholar]
- (12).Imazato S, Imai T, Russell RR, Torii M, Ebisu S. J. Biomed. Mater. Res. 1998;39:511. doi: 10.1002/(sici)1097-4636(19980315)39:4<511::aid-jbm1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- (13).Imazato S, Torii M, Tsuchitani Y, McCabe JF, Russell RR. J. Dent. Res. 1994;73:1437. doi: 10.1177/00220345940730080701. [DOI] [PubMed] [Google Scholar]
- (14).Hayashi K, Saito N, Sugimura H, Takai O, Nakagiri N. Ultramicroscopy. 2002;91:151. doi: 10.1016/s0304-3991(02)00094-3. [DOI] [PubMed] [Google Scholar]
- (15).Seah MP, Dench WA. Surf. Interface Anal. 1979;1:2. [Google Scholar]
- (16).Katsikogianni M, Missirlis YF. Eur. Cell Mater. 2004;8:37. doi: 10.22203/ecm.v008a05. [DOI] [PubMed] [Google Scholar]
- (17).van Noort R. Introduction to Dental Materials. 2nd ed. Mosby; Edinburgh; New York: 2002. [Google Scholar]
- (18).Weerkamp AH, van der Mei HC, Busscher HJ. J. Dent. Res. 1985;64:1204. doi: 10.1177/00220345850640100501. [DOI] [PubMed] [Google Scholar]
- (19).Busscher HJ, van der Mei HC. Adv. Dent. Res. 1997;11:24. doi: 10.1177/08959374970110011301. [DOI] [PubMed] [Google Scholar]
- (20).Bollen CM, Papaioanno W, Van Eldere J, Schepers E, Quirynen M, van Steenberghe D. Clin. Oral Implants Res. 1996;7:201. doi: 10.1034/j.1600-0501.1996.070302.x. [DOI] [PubMed] [Google Scholar]
- (21).Quirynen M, Bollen CM, Papaioannou W, Van Eldere J, van Steenberghe D. Int. J. Oral Maxillofac. Implants. 1996;11:169. [PubMed] [Google Scholar]
- (22).Quirynen M, van der Mei HC, Bollen CM, Schotte A, Marechal M, Doornbusch GI, Naert I, Busscher HJ, van Steenberghe D. J. Dent. Res. 1993;72:1304. doi: 10.1177/00220345930720090801. [DOI] [PubMed] [Google Scholar]
- (23).Tang H, Cao T, Liang X, Wang A, Salley SO, McAllister J, 2nd, Ng KY. J. Biomed. Mater. Res. A. 2009;88:454. doi: 10.1002/jbm.a.31788. [DOI] [PubMed] [Google Scholar]
- (24).Mitik-Dineva N, Wang J, Mocanasu RC, Stoddart PR, Crawford RJ, Ivanova EP. Biotechnol. J. 2008;3:536. doi: 10.1002/biot.200700244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.