Abstract
A fluorescent nucleobase analogue, 7-aminoquinazoline-2,4-(1H,3H)-dione, is incorporated into a DNA oligonucleotide and senses mismatched pairing by displaying G-specific fluorescence enhancement.
Single nucleotide polymorphisms (SNPs),1 mutated base pairs, have been linked to specific diseases or susceptibility to particular therapeutics.2 While there are several developed and commercialized approaches for detecting SNPs,3 many recent advancements have centered around the design of base-discriminating fluorescent nucleosides.4–7 Following incorporation into DNA hybridization probes and duplex formation with target oligonucleotides, the emissive nucleosides display characteristic photophysical signature, depending on their pairing partner.4,8
To develop base discriminating probes, it is important to identify heterocycles that are structurally similar to native nucleobases and capable of Watson–Crick pairing. Red shifted absorption spectra relative to native nucleosides, permitting selective excitation, are highly desirable. The emission of the fluorescent analogs should be sensitive to its hybridization microenvironment, and perhaps more importantly, fluorescence enhancement rather than quenching should be associated with positive identification of a mismatch. Detecting mismatched G residues has, therefore, presented a challenge, as guanine, being the easiest nucleobase to oxidize,9–10 frequently quenches the emission of most commonly used fluorophores.11–14 Here we present a new fluorescent pyrimidine analog that, when hybridized against G, displays an enhanced emission when compared to a perfect duplex or all other mismatches.
In accordance with our design principles,5–7 we have synthesized a polarizable nucleobase, 7-aminoquinazoline-2,4-(1H,3H)-dione 1 and the corresponding 2′-deoxynucleoside 2, which contain an electron-rich ring fused into an electron-deficient pyrimidine (Scheme 1). We surmise that placing the electron donating amine group in a conjugated position to the pyrimidine’s carbonyl would facilitate a charge transfer transition and greater sensitivity of the photophysical characteristics to environmental changes. To assess the nucleoside’s sensitivity to its microenvironment, its absorption and emission spectra were recorded in solvents of distinct polarity (Fig. 1 and Table 1). Solvent polarity has little effect on the lowest energy absorption maximum of nucleoside 2 (316 ± 1 nm), but the absorption band around 288 nm is sensitive to polarity changes, resulting in a greater molar absorptivity in nonpolar solvents.
Scheme 1.
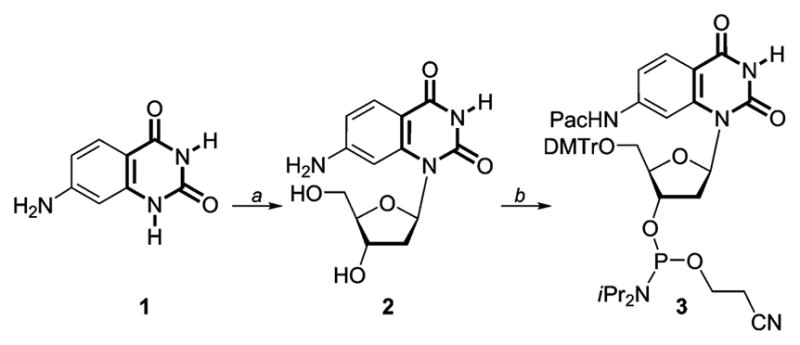
Synthesis of the nucleoside and phosphoramidite based on 7-aminoquinazoline-2,4-(1H,3H)-dione. Reagents: (a) (i) (NH4 )2 SO4, N, O-bis(trimethylsilyl)acetamide, CF3 SO3 Si(CH3 )3,2-D-3,5-di-O-p-toluoyl-α-L-erythro-pentofuranosyl chloride, CH3 CN; (ii) conc. NH4 OH, 40%. (b) (i) (CH3 )3 SiCl, phenoxyacetic anhydride, H2 O, conc. NH4 OH, pyridine, 75%; (ii) DMTrCl, Et3 N, pyridine, 85%; (iii) iPr2 NEt, (iPr2 N)P(Cl)O-CH2 CH2 CN, ClCH2 CH2 Cl, 65%.15
Fig. 1.
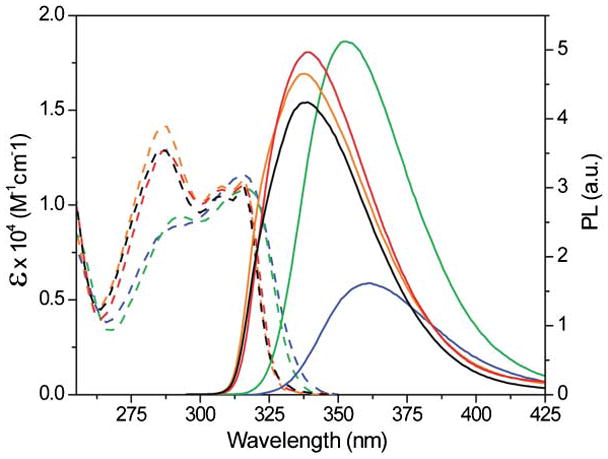
Absorption (—) and emission (—) spectra of nucleoside 2 in water (blue), methanol (green), acetonitrile (red), dioxane (orange), and dichloromethane (black).
Table 1.
Photophysical data of nucleoside 2a
| Solvent | ET (30)b | λbsc/nm | λem/nm | Ireld |
|---|---|---|---|---|
| Water | 65.3 | 316 | 361 | 1.0 |
| Methanol | 55.7 | 316 | 352 | 3.2 |
| Acetonitrile | 45.9 | 316 | 339 | 3.1 |
| Dichloromethane | 40.9 | 316 | 338 | 2.6 |
| Dioxane | 36.4 | 316 | 336 | 2.9 |
Conditions for absorption and emission spectra: 5.0 and 0.5 × 10−5 M, respectively.
Units are kcal mol−1.
The lowest energy maximum is given.
Relative emission intensity with respect to intensity in water.
Importantly, both emission wavelength and intensity are affected by solvent polarity. In water, the most polar solvent examined, 2 exhibits the most quenched and bathochromically shifted emission band (Fig. 1), peaking around 361 nm (ΦF = 0.039 ± 0.006, Stoke Shift = 3.9 × 103 cm−1). In methanol, nucleoside 2 displays the most intense emission with an emission band at 352 nm (ΦF = 0.14 ± 0.01, Stoke Shift = 3.2 × 103 cm−1). In solvents of lower polarity, 2 shows more hyperchromically shifted emission with decreasing intensity (Table 1, Stoke Shifts = 1.9–2.1 × 103 cm−1). These observations suggest an enlarged dipole and charge transfer character of the excited state when compared to the ground state.
To incorporate the non native nucleoside into a DNA oligonucleotide, phosphoramidite 3 was prepared (Scheme 1). 7-Aminoquinazoline-2,4(1H,3H)-dione 1 was glycosylated to provide the modified nucleoside 2 after saponification of all esters and isolation of the β-anomer (X-ray Structure: Figure S1 and Table S1†).15 Protection of the 5′-hydroxyl as the 4,4′-dimethoxytrityl (DMTr) derivative, followed by phosphitylation of the 3′-hydroxyl, provided phosphoramidite 3 (Scheme 1). Standard solid-phase oligonucleotide synthesis was utilized to prepare the 13-mer DNA construct 4, where probe 2 was placed in the middle of the sequence (Fig. 2). The oligonucleotide was purified by PAGE, and MALDI-TOF mass spectrometry confirmed its full length and the presence of the intact emissive nucleoside 2 (Figure S2†).15
Fig. 2.
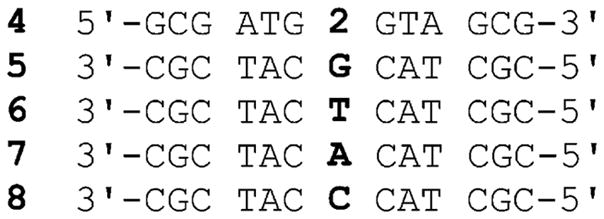
Synthesized oligonucleotide 4 and oligonucleotides used in hybridization and fluorescence experiments.
The fluorescent single strand DNA oligonucleotide 4 exhibits a similar, albeit broader, emission profile to the nucleoside in water with an emission band around 361 nm. Upon hybridization to its complement 7, a quenched emission at 363 nm is observed (Fig. 3 and Table 2). In contrast, when the fluorescently labeled DNA oligonucleotide 4 is hybridized with 5, an oligonucleotide with a G mismatch opposite nucleoside 2, its emission is greatly enhanced and hyperchromically shifted to 353 nm, displaying an emission more similar to nucleoside 2 in methanol (Fig. 3 and Table 2). Other oligonucleotides with mismatches (6 and 8) failed to produce a dramatic increase in fluorescence intensity and all displayed emission bands around 362 nm, where nucleoside 2 emits in water. Importantly, thermal denaturation measurements (Table 2 and Figure S4†),15 determined by monitoring changes in absorbance at 260 nm as a function of temperature, show that stable duplexes were formed for all oligonucleotide pairs. The T m value for the complemented duplex 4·7 (T m = 57 ± 1 °C) was within error of the melting temperature of an unmodified control duplex (Tm = 58 ± 1 °C) (Figure S3–S4†). Hybridization with DNA strands containing mismatches do show, as expected, destabilization (Table 2).
Fig. 3.
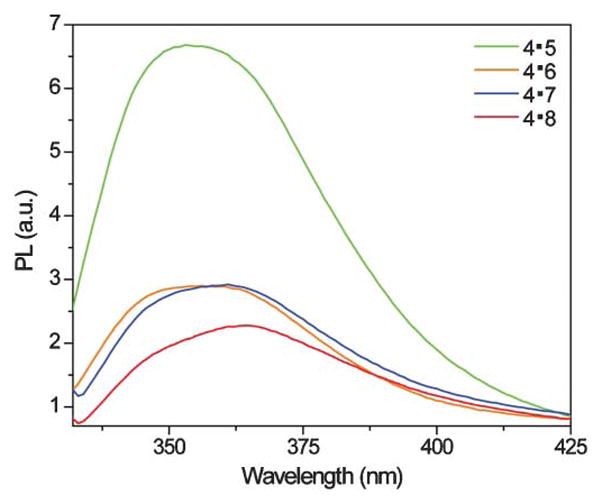
Emission spectra of 4·5 (green), 4·6 (orange), 4·7 (blue), and 4·8 (red). Conditions same as listed in Table 2.
Table 2.
Photophysical data of oligonucleotide 4 and its duplexesa
| Duplexes | 4·5 | 4·6 | 4·7 | 4·8 |
|---|---|---|---|---|
| λem/nm | 353 | 362 | 361 | 365 |
| Irelb | 2.2 | 1.3 | 1.0 | 0.8 |
| TM °C−1 | 50 ± 1 | 51 ± 1 | 57 ± 1 | 50 ± 1 |
Conditions: 5.0 × 10−6 M in 2.0 × 10−2M Na3 PO4, pH 7.0.
Relative emission intensity with respect to intensity of 4·7.
Nucleoside 2 uniquely reports the presence of a G mismatch with over a two-fold enhanced emission, compared to its emission intensity in a perfect duplex when found opposite A, a feature rarely seen with isosteric/isomorphic fluorescent nucleoside analogs.11–14 While the underlying molecular factors governing this behavior are unclear at present, a disparity between the redox potential of G and the new nucleobase, coupled to environmental factors influencing the solvation of the modified base are likely to be influencing factors. It is tempting to speculate that a formation of a wobble G·2 pair anchors the emissive nucleoside in a partially exposed geometry, while still preserving a partially stacked and desolvated microenvironment.16–18 Regardless of these putative structural features, the results reported here demonstrate that new emissive nucleobase analogs can display unique photophysical features and potentially find utility for mismatch detection.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health for their generous support (GM 069773), the National Science Foundation (instrumentation grant CHE-0741968), and Nicholas Greco for his assistance with MALDI experiments.
Footnotes
Electronic supplementary information (ESI) available: Details of synthesis, characterization of compounds, thermal melting data and additional fluorescence data. CCDC reference number 784395. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0ob00413h
Notes and references
- 1.(a) Brookes AJ. Gene. 1999;234:177. doi: 10.1016/s0378-1119(99)00219-x. [DOI] [PubMed] [Google Scholar]; (b) Chakravarti A. Nat Genet. 1999;21:56. doi: 10.1038/4482. [DOI] [PubMed] [Google Scholar]; (c) Haga H, Yamada Y, Ohnishi Y, Nakamura Y, Tanaka T. J Hum Genet. 2002;47:605. doi: 10.1007/s100380200092. [DOI] [PubMed] [Google Scholar]; (d) The International HapMap Consortium Nature 2005437129916255080 [Google Scholar]
- 2.(a) McCarthy JJ, Hilfiker R. Nat Biotechnol. 2000;18:505. doi: 10.1038/75360. [DOI] [PubMed] [Google Scholar]; (b) Pastinen T, Hudson TJ. Science. 2004;306:647. doi: 10.1126/science.1101659. [DOI] [PubMed] [Google Scholar]
- 3.(a) Paris PL, Langenhan JM, Kool ET. Nucleic Acids Res. 1998;26:3789. doi: 10.1093/nar/26.16.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Carlson CS, Newman TL, Nickerson DA. Curr Opin Chem Biol. 2001;5:78. doi: 10.1016/s1367-5931(00)00171-x. [DOI] [PubMed] [Google Scholar]; (c) Crockett AO, Wittwer CT. Anal Biochem. 2001;290:89. doi: 10.1006/abio.2000.4957. [DOI] [PubMed] [Google Scholar]; (d) Kwok PY. Annu Rev Genomics Hum Genet. 2001;2:235. doi: 10.1146/annurev.genom.2.1.235. [DOI] [PubMed] [Google Scholar]; (e) Twyman RM. Curr Top Med Chem. 2004;4:1421. doi: 10.2174/1568026043387656. [DOI] [PubMed] [Google Scholar]; (f) Nakatani K. ChemBioChem. 2004;5:1623. doi: 10.1002/cbic.200400161. [DOI] [PubMed] [Google Scholar]; (g) Suh Y, Cantor C. Mutat Res, Fundam Mol Mech Mutagen. 2005;573:1. doi: 10.1016/j.mrfmmm.2005.01.003. [DOI] [PubMed] [Google Scholar]; (h) Sobrino B, Brion M, Carracedo A. Forensic Sci Int. 2005;154:181. doi: 10.1016/j.forsciint.2004.10.020. [DOI] [PubMed] [Google Scholar]; (i) Valis L, Amann N, Wagenknecht HA. Org Biomol Chem. 2005;3:36. doi: 10.1039/b414672g. [DOI] [PubMed] [Google Scholar]; (j) Asseline U, Chassignol M, Aubert Y, Roig V. Org Biomol Chem. 2006;4:1949. doi: 10.1039/b602262f. [DOI] [PubMed] [Google Scholar]; (k) Friedrich A, Hoheisel JD, Marmé N, Knemeyer JP. FEBS Lett. 2007;581:1644. doi: 10.1016/j.febslet.2007.03.031. [DOI] [PubMed] [Google Scholar]; (l) Kumar TS, Wengel J, Hrdlicka PJ. ChemBioChem. 2007;8:1122. doi: 10.1002/cbic.200700144. [DOI] [PubMed] [Google Scholar]; (m) Ergen E, Weber M, Jacob J, Herrmann A, Müllen K. Chem–Eur J. 2006;12:3707. doi: 10.1002/chem.200501526. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto A, Saito Y, Saito I. J Photochem Photobiol, C. 2005;6:108. [Google Scholar]
- 5.Sinkeldam RW, Greco NJ, Tor Y. Chem Rev. 2010;110:2579. doi: 10.1021/cr900301e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greco NJ, Tor Y. J Am Chem Soc. 2005;127:10784. doi: 10.1021/ja052000a. [DOI] [PubMed] [Google Scholar]
- 7.Tor Y. Tetrahedron. 2007;63:3425. [Google Scholar]
- 8.(a) Tainaka K, Tanaka K, Ikeda S, Nishiza KI, Unzai T, Fujiwara Y, Saito I, Okamoto A. J Am Chem Soc. 2007;129:4776. doi: 10.1021/ja069156a. [DOI] [PubMed] [Google Scholar]; (b) Saito Y, Mizuno E, Bag SS, Suzuka I, Saito I. Chem Commun. 2007:4492. doi: 10.1039/b709715h. [DOI] [PubMed] [Google Scholar]; (c) Takei F, Suda H, Hagihara M, Zhang J, Kobori A, Nakatani K. Chem–Eur J. 2007;13:4452. doi: 10.1002/chem.200601496. [DOI] [PubMed] [Google Scholar]; (d) Hudson RHE, Ghorbani-Choghamarani A. Org Biomol Chem. 2007;5:1845. doi: 10.1039/b705805e. [DOI] [PubMed] [Google Scholar]; (e) Ryu JH, Seo YJ, Hwang T, Lee JY, Kim BH. Tetrahedron. 2007;63:3538. [Google Scholar]; (f) Xiao Q, Ranasinghe RT, Tang AMP, Brown T. Tetrahedron. 2007;63:3483. [Google Scholar]; (g) Srivatsan SG, Weizman H, Tor Y. Org Biomol Chem. 2008;6:1334. doi: 10.1039/b801054d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kittler L, Lober G, Gollmick FA, Berg H. Bioelectrochem Bioenerg. 1980;7:503. [Google Scholar]
- 10.Xie H, Yang D, Heller A, Gao Z. Biophys J. 2007;92:L70. doi: 10.1529/biophysj.106.102632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seidel CAM, Schulz A, Sauer MHM. J Phys Chem. 1996;100:5541. [Google Scholar]
- 12.Dohno C, Saito I. ChemBioChem. 2005;6:1075. doi: 10.1002/cbic.200400325. [DOI] [PubMed] [Google Scholar]
- 13.Behlke MA, Huang L, Bogh L, Rose S, Devor EJ. Fluorescence Quenching by Proximal G-bases. Integrated DNA Technologies; 2005. [Google Scholar]
- 14.(a) Wang W, Chen C, Qian MX, Zhao XS. Sens Actuators, B. 2008;129:211. [Google Scholar]; (b) Mizuta M, Seio K, Ohkubo A, Sekine M. J Phys Chem B. 2009;113:9562. doi: 10.1021/jp807562c. [DOI] [PubMed] [Google Scholar]
- 15.See supporting information for additional details.
- 16.Rabinovich D, Haran T, Eisenstein M, Shakked Z. J Mol Biol. 1988;200:151. doi: 10.1016/0022-2836(88)90340-3. [DOI] [PubMed] [Google Scholar]
- 17.Kalnik MW, Kouchakdjian M, Li BFL, Swann PF, Patel DJ. Biochemistry. 1988;27:108. doi: 10.1021/bi00401a018. [DOI] [PubMed] [Google Scholar]
- 18.Modrich P. Annu Rev Biochem. 1987;56:435. doi: 10.1146/annurev.bi.56.070187.002251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


