Abstract
Background
Calcineurin inhibitor nephrotoxicity in nonrenal allograft recipients can lead to end-stage renal disease and the need for kidney transplantation. We sought to evaluate the role of alemtuzumab induction in this population.
Patients and Methods
We evaluated 144 patients undergoing kidney transplantation after nonrenal transplantation between May 18, 1998, and October 8, 2007. Seventy-two patients transplanted between January 15, 2003, and October 8, 2007, received alemtuzumab induction and continued their pretransplant immunosuppression. Seventy-two patients transplanted between May 18, 1998, and July 21, 2007, did not receive alemtuzumab induction, but received additional steroids and maintenance immunosuppression. Donor and recipient demographics were comparable.
Results
Overall, 1- and 3-year patient survival and renal function were comparable between the two groups. One- and 3-year graft survival was 93.0% and 75.3% in the alemtuzumab group and 83.3% and 68.7% in the no alemtuzumab group, respectively (P=0.051). The incidence of acute rejection was lower in the alemtuzumab group, 15.3%, than in the no alemtuzumab group, 41.7% (P=0.0001). The incidence of delayed graft function was lower in the alemtuzumab group, 9.7%, than in the no alemtuzumab group, 25.0% (P=0.003). The incidence of viral complications was comparable.
Conclusion
Alemtuzumab induction with simple resumption of baseline immunosuppression in patients undergoing kidney transplantation after nonrenal transplantation represents a reasonable immunosuppressive strategy.
Keywords: Kidney, Nonrenal, Alemtuzumab
In patients undergoing nonrenal transplantation, the favorable outcomes associated with calcineurin inhibitors (CNI) have been tempered by the negative impact of CNI nephrotoxicity (1). This well-described phenomenon has led to the development of end-stage renal disease as an important complication of nonrenal transplantation, and some of these patients have gone on to kidney transplantation. A number of centers have reported on the efficacy of alemtuzumab induction or preconditioning in patients undergoing kidney transplantation alone (2-10). However, there are no publications describing the utility of alemtuzumab in patients undergoing kidney transplantation after nonrenal transplantation. This report discusses our single-center, retrospective experience with alemtuzumab induction and compares it to a previous cohort not receiving alemtuzumab.
PATIENTS AND METHODS
Between May 18, 1998, and October 8, 2007, 144 patients underwent kidney transplantation after nonrenal transplantation (Table 1). Seventy-two patients received alemtuzumab induction (one dose of 30 mg intravenously or 0.4–0.5 mg/kg in pediatric patients), with two perioperative doses of steroids, and simple resumption of the prekidney transplantation immunosuppressive regimen. Seventy-two patients did not receive alemtuzumab; they routinely received additional induction and maintenance steroids, higher doses of CNIs, and the addition of an antiproliferative agent (mycophenolate mofetil) if they had not been on the one previously; in addition, three patients received thymoglobulin, and 10 received daclizumab induction. There were 133 (92.4%) adults and 11 (7.6%) children. Thirty-five (24.3%) had undergone previous heart, 16 (11.1%) lung, 87 (60.4%) liver, and 6 (4.2%) multivisceral transplantation. There were 100 (69.4%) deceased donor transplants, with a mean cold ischemia time of 24.7±7.9 hr, and 44 (30.6%) living donor cases; although there was a slightly higher percentage of living donors in the alemtuzumab group compared with that of the no alemtuzumab group, this was not statistically different. Alemtuzumab began to be used in our institution in late 2002; hence, the follow-up for the alemtuzumab patients was shorter, 23.3 ± 15.0 months, than for the no alemtuzumab patients, 48.1 ± 36.9 months. Once alemtuzumab began to be used, almost all patients undergoing kidney transplantation after nonrenal transplantation received it, except for one patient who received thymoglobulin and six patients who received daclizumab. The overall mean follow-up was 35.7±30.7 months.
TABLE 1.
Recipients and donor demographics
| Overall | Alemtuzumab group | No alemtuzumab group | |
|---|---|---|---|
| Time | From May 18, 1998, to October 8, 2007 | From January 15, 2003, to October 8,2007 | From May 18, 1998 to July 21, 2007 |
| N | 144 | 72 | 72 |
| Recipient age (yr) | 52.1±16.6 | 54.1±15.5 | 50.1±17.5 |
| Donor age (yr) | 38.4±16.5 | 38.0±15.5 | 38.9±17.6 |
| Time after nonrenal Tx (yr) | 8.1±4.7 | 8.3±5.1 | 8.0±4.4 |
| Adult, n (%) | 133 (92.4) | 68 (94.4) | 65 (90.3) |
| Child, n (%) | 11 (7.6) | 4 (5.6) | 7 (9.7) |
| Previous | |||
| Heart, n (%) | 35 (24.3) | 26 (36.1) | 9 (12.5) |
| Lung, n (%) | 16 (11.1) | 7 (9.7) | 9 (12.5) |
| Liver, n (%) | 87 (60.4) | 37 (51.4) | 50 (69.4) |
| Multivisceral, n (%) | 6 (4.2) | 2 (2.8) | 4 (5.6) |
| Deceased donor, n (%) | 100 (69.4) | 45 (62.5) | 55 (76.4) |
| Cold ischemia time (hr) | 24.7±7.9 | 24.2±7.5 | 25.1±8.4 |
| HCV+, n (%) | 19 (13) | 7 (10) | 12 (17) |
| Living donor, n (%) | 44 (30.6) | 27 (37.5) | 17 (23.6) |
| PRA | 3.3±9.6 | 2.6±9.7 | 4.0±9.4 |
Tx, transplantation; HCV+, hepatitis C virus positive; PRA, panel reactive antibody.
Statistics
Continuous variables were compared using the t test with Levene’s test used for verifying the assumption of equality of variance. The chi-square test was used to compare categorical variables.
Institutional Oversight
The data analysis was performed on deidentified data by one of the honest brokers in our division, Joseph Donaldson, under the guidelines of the Institutional Review Board protocol number 0505123 (11).
RESULTS
Overall, 1- and 3-year actuarial patient survival was 91.5% and 75.3%, and it was 93.0% and 78.9% in the alemtuzumab group and 90.0% and 72.4% in the no alemtuzumab group, respectively (P=ns). Overall, 1- and 3-year actuarial graft survival was 88.1% and 71.4% and it was 93.0% and 75.3% in the alemtuzumab group and 83.3% and 68.7% in the no alemtuzumab group, respectively (P=0.051, Fig. 1; Table 2). The overall mean serum creatinine levels at 1 and 3 years were 1.4±0.7 and 1.5±0.9 mg/dL, respectively, and were not statistically different between the two groups. The incidence of acute rejection was lower in the alemtuzumab group, 15.3%, than in the no alemtuzumab group, 41.7% (P=0.0001, Table 3). The incidence of delayed graft function, defined as the need for dialysis during the first week after transplantation, was lower in the alemtuzumab group, 9.7%, than in the no alemtuzumab group, 25.0% (P=0.003, Table 3). This difference persisted only when the deceased donor cases were considered: the incidence of delayed graft function in the alemtuzumab group was 15.6% and in the no alemtuzumab group, it was 32.7% (P<0.05). The incidence of viral complications was not different between the two groups. We performed several subgroup analyses, looking for any other significant factors, including living donation, hepatitis C, diabetes, and the use of extended criteria donor kidneys, which might have explained the differences, but none was associated with any outcome differences (data not shown).
FIGURE 1.
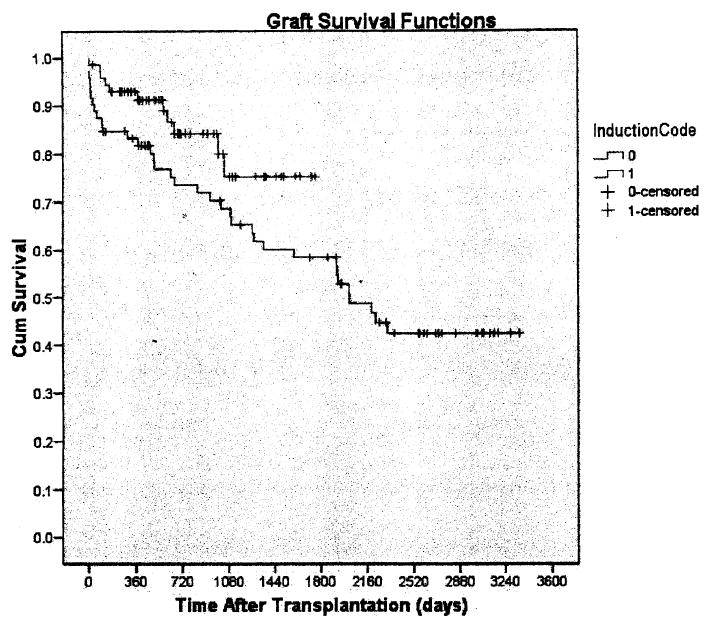
Graft survival in kidney transplantation after nonrenal transplantation (alemtuzumab; no alemtuzumab).
TABLE 2.
Results
| Overall | Alemtuzumab group | No alemtuzumab group | |
|---|---|---|---|
| Patient survival (%) | |||
| 1 yr | 91.5 | 93.0 | 90.0 |
| 3 yr | 75.3 | 78.9 | 72.4 |
| Graft survival (%) | |||
| 1 yr | 88.1 | 93.0 | 83.3 |
| 3 yr | 71.4 | 75.3* | 68.7 |
| Mean serum creatinine (mg/dL) | |||
| 1 yr | 1.4±0.7 | 1.3±0.5 | 1.5±0.8 |
| 3 yr | 1.5±0.9 | 1.3±0.7 | 1.6±1.0 |
P=0.051.
TABLE 3.
Complications
| Overall, % | Alemtuzumab group, % | No alemtuzumab group, % | |
|---|---|---|---|
| Complications | |||
| Acute rejection | |||
| 6 mo | 16 | 2.8 | 29.2** |
| l yr | 20.8 | 8.3 | 33.3*** |
| Total | 28.5 | 15.3 | 41.7** |
| Delayed graft function | 17.4 | 9.7 | 25.0*** |
| Living donor | 0 | 0 | 0 |
| Deceased donor | 25 | 15.6 | 32.7**** |
| CMV | 0 | 0 | 0 |
| PTLD | 0.7 | 0 | 1.4 |
| BK virus | 4.2 | 4.2 | 2.8 |
P=0.0001;
P=0.003;
P<0.05.
CMV, cytomegalovirus; PTLD, posttransplant lymphoproliferative disorders.
There were 19 hepatitis C virus (HCV) positive patients undergoing kidney transplantation after nonrenal transplantation: 7 (4 liver, 2 heart, and 1 lung) received alemtuzumab and 12 (all liver) did not, 10 received no induction and two received daclizumab. The alemtuzumab cases were transplanted before the publication of the article, which showed problematic outcomes associated with alemtuzumab and HCV in liver transplantation (12). The numbers of cases were in any event too small to analyze.
The alemtuzumab and no alemtuzumab differences were observed in all nonrenal transplant subgroups (i.e., heart, lung, liver, and multivisceral—data not shown), although statistical significance was noted only when the groups were combined.
DISCUSSION
Kidney after nonrenal transplantation is an uncommon subject for discussion, and the approach to immunosuppression is not well defined. In our center, it has accounted for 7.1% of the kidney transplantations that have been performed, with 144/2034 cases in less than 10 years. AS the kidney is a third-party antigen, and as the level of immunosuppression in nonrenal transplant recipients tends to be relatively low by the time a kidney transplantation needs to be performed, some additional immunosuppression needs to be administered to prevent rejection of the kidney. The advantage of alemtuzumab induction in this context is that the baseline immunosuppression does not need to be changed. This simplifies patient management after transplantation and further may have the advantage of being associated with less rejection, less delayed graft function, and slightly better graft survival, without any increase in viral complications. However, it is important to remember that the no alemtuzumab group was not randomized and was more of an historic control; hence, these differences have to be interpreted with caution.
There are certain settings in kidney after nonrenal transplantation where alemtuzumab may not necessarily be a good idea. These would include patients who are HCV positive and have had a previous liver transplant (12), or recently transplanted patients who have received heavy immunosuppression for the nonrenal organ. In these situations, accounting for six cases in our series, we used daclizumab (1 mg/kg) induction at the time of transplantation and every 2 weeks for four additional doses, with standard tacrolimus/mycophenolate mofetil-based immnosuppression, without additional maintenance steroids. This seemed anecdotally to be a satisfactory approach in these six patients.
This experience has important and obvious limitations. It is retrospective, and, as mentioned earlier, not randomized, and the no alemtuzumab group is mostly an historical control. Unfortunately, kidney transplantation after nonrenal transplantation is not performed often, and a randomized trial, single-center or multicenter, while desirable, will not be straightforward to perform. In the absence of such a trial, the experience reported here suggests that alemtuzumab induction with resumption of prekidney transplantation immunosuppression may possibly represent a simple and effective regimen in patients undergoing kidney transplantation after nonrenal transplantation.
Acknowledgments
The authors thank Deborah Good, R.N., B.S.N., C.C.T.C.; Gemi James, R.N., C.C.T.C.; Angela Barber, R.N., B.S., C.C.T.C.; Janice Glidewell, R.N., B.S.N., C.C.T.C.; Mitzi Barker, R.N., C.C.T.C.; Corde McFeaters, R.N., B.S.N., C.C.T.C.; Kim Meyer, R.N.; Stacy Acevedo, R.N., C.C.T.C.; Nancy Eger, R.N., B.S.N.; Alice Maglione, R.N., A.D.; Mark Paynter, B.S.N., R.N., C.C.T.C.; Maureen Vekasy, R.N., C.C.T.C.; Amy Singh, R.N., B.S.N., C.C.T.C.; Lori Prothero, R.N.; Diane Connors, R.N., M.P.H.; Tim Donovan, R.N., C.C.T.C.; Melissa Choma, R.N., C.C.R.N.; Hollie Lambert R.N.; Michelle Coombs, PA.-C.; Shannon Ross, P.A.-C.; Ashley Myers, PA.-C.; and Kris Schonder, PharmD., for their preoperative and postoperative care of the patients, and Melissa Connell for manuscript preparation.
Research and design: R.S., T.E.S.; writing of the manuscript: R S.; performance of research. RS., A.B., H.P.T., C.M., V.S., D.B., P.S.R, J.M.C., D.E., J.W.M., S.W., G.K., K.RM.C., K.A.-E., G.M.; and data analysis: RS., LD.
References
- 1.Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:331. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 2.Waldmann H. A personal history of the CAMPATH-1H antibody. Med Oncol. 2002;19(suppl):S3. doi: 10.1385/mo:19:2s:s03. [DOI] [PubMed] [Google Scholar]
- 3.Calne R, Friend P, Moffatt S, et al. Prope tolerance, perioperative Campath lH, and low- dose cyclosporine monotherapy in renal allograft recipients. Lancet. 1998;351:1701. doi: 10.1016/S0140-6736(05)77739-4. [DOI] [PubMed] [Google Scholar]
- 4.Stuart FP, Leventhal JR, Kaufman DB. Alemtuzumab facilitates prednisone free immunosuppression in kidney transplant recipients with no early rejection. Am J Transplant. 2002;2(suppl):397. doi: 10.1034/j.1600-6143.2002.20715.x. [DOI] [PubMed] [Google Scholar]
- 5.Knechtle SJ, Pirsch JD, Fechner HJ, Jr, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation results of a pilot study. Am J Transplant. 2003;3:722. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- 6.Kirk AD, Hale DA, Mannon RB, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H) Transplantation. 2003;76:120. doi: 10.1097/01.TP.0000071362.99021.D9. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro R, Basu A, Tan HP, et al. Kidney transplantation under minimal immunosuppression after pretransplant lymphoid depletion with thymoglobulin or Campath. J Am Coll Surg. 2005;200:505. doi: 10.1016/j.jamcollsurg.2004.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro R, Ellis D, Tan HP, et al. Antilymphoid antibody preconditioning and tacrolimus monotherapy for pediatric kidney transplantation. J Pediatr. 2006;148:813. doi: 10.1016/j.jpeds.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan HP, Kaczorowski DJ, Basu A, et al. Living donor renal transplantation using alemtuzumab induction and tacrolimus monotherapy. Am J Transpl. 2006;6:2409. doi: 10.1111/j.1600-6143.2006.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro R, Zeevi A, Basu A, et al. Alemtuzumab preconditioning with tacrolimus monotherapy—The impact of serial monitoring for donor-specific antibody. Transplantation. 2008;85:1125. doi: 10.1097/TP.0b013e31816a8a6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.University of Pittsburgh Institutional Review Board (homepage on the internet) Pittsburgh: University of Pittsburgh; 2006. [February 10, 2006]. Jurisdiction, Structure, and Responsibilities of the Institutional Review Board. Reference Manual for the Use of Human Subjects in Research. Available at: http://www.irb.pitt.edu/manual/preface.pdf. [Google Scholar]
- 12.Eghtesad B, Fung JJ, Demetris AJ, et al. Immunosuppression for liver transplantation in HCV-infected patients: Mechanism-based principles. Liver Transpl. 2005;11:1343. doi: 10.1002/lt.20536. [DOI] [PMC free article] [PubMed] [Google Scholar]


