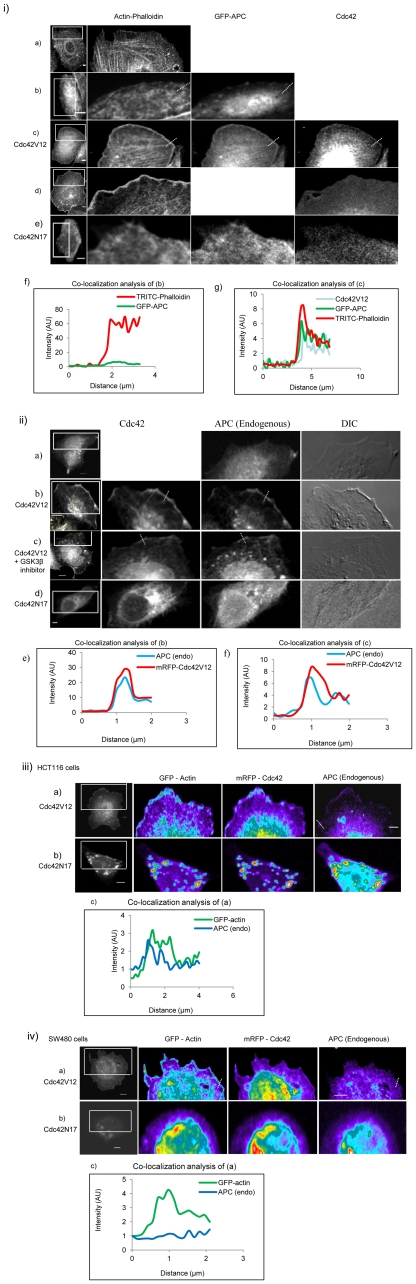
Figure 5. Leading edge localization of APC and Cdc42V12.
(i) APC and Cdc42V12 colocalize with actin at the leading edges in CHO cells. Cells were stained with TRITC-phalloidin for actin and anti-HA for Cdc42 with Alexa 405-tagged secondary antibody. (a) untransfected CHO cell, (b) cell expressing GFP-APC, (c) cell coexpressing GFP-APC and HA-Cdc42V12, (d) cell expressing mRFP-Cdc42V12 and (e) cell expressing GFP-APC and HA-Cdc42N17. Image processing for leading edge localization was done using ImageJ software. Colocalization analysis was carried out along the white dotted lines as shown on the images. (f) Line intensity colocalization analysis of GFP-APC and TRITC-phalloidin of the cell shown in panel (b) and (g) line intensity colocalization analysis of GFP-APC, HA-Cdc42V12 and TRITC-phalloidin of the cell shown in panel (c). Scale bar = 5 µm. (ii) Endogenous APC colocalizes with Cdc42V12 at the leading edges in HCT116 cells. (a) Cell stained for endogenous APC with anti-APC primary antibody (ab-2) and Alexa 488-tagged secondary antibody, (b) cell expressing mRFP-Cdc42V12 and stained for endogenous APC, (c) mRFP-Cdc42V12 expressing cell was treated with GSK-3β inhibitor (cell-permeable PKC ζ–specific pseudosubstrate) followed by immunostaining for APC, (d) cell expressing mRFP-Cdc42N17 stained for endogenous APC. Image processing for leading edge localization was done by applying a low pass filter in Metamorph software to subtract out-of-focus blur. Colocalization analysis was carried out along the white dotted lines shown on the images. (e) Line intensity colocalization analysis of endogenous APC and mRFP-Cdc42V12 of the cell shown in panel (b) and (f) line intensity colocalization analysis of endogenous APC and mRFP-Cdc42V12 of the cell shown in panel (c). Scale bar = 5µm. (iii) Endogenous APC colocalization with GFP-actin in HCT116 cells in presence of Cdc42V12 or Cdc42N17. (a) Cell coexpressing mRFP-Cdc42V12 and GFP-actin. (b) Cell coexpressing mRFP-Cdc42N17 and GFP-actin. Image processing for leading edge localization was done by applying a low pass filter in Metamorph software to subtract out-of-focus blur. Pseudo-coloring was applied using Metamorph software. Colocalization analysis was carried out along the white dotted lines shown on the images. (c) Line intensity colocalization analysis of stained endogenous APC and GFP-actin of cell shown in panel (a). Scale bar = 5 µm. (iv) Endogenous APC colocalization with GFP-actin in SW480 cells in presence of Cdc42V12 or Cdc42N17. (a) Cell coexpressing mRFP-Cdc42V12 and GFP-actin, (b) cell coexpressing mRFP-Cdc42N17 and GFP-actin. Image processing for leading edge localization was done by applying a low pass filter in Metamorph software to subtract out-of-focus blur. Pseudo-coloring was applied using Metamorph software. Colocalization analysis was carried out along the white dotted lines shown on the images. (c) Line intensity colocalization analysis of stained endogenous APC and GFP-actin of cell shown in panel (a). Scale bar = 5µm.