Abstract
Study design
Controlled laboratory study using a repeated measures approach.
Objective
To quantify the amount of strain on cadaver posterior shoulder tissues during simulated clinical tests across different tissue conditions.
Background
Several clinical tests are used to quantify posterior glenohumeral joint (GHJ) tissue tightness; however the ability of these tests to directly assess the flexibility or tightness of the posterior capsule has not been evaluated.
Methods
The middle and lower regions of the posterior shoulder tissues were instrumented with strain gauges on 8 cadaver shoulder specimens. Strain was quantified on the posterior shoulder muscles, on the native posterior GHJ capsule (baseline condition), and on the posterior GHJ capsule after it was experimentally contracted using thermal energy. Five simulated clinical tests were compared across each of the 3 conditions; humerus cross-body adduction, and GHJ internal rotation with the humerus positioned in 4 combinations of plane and elevation angle. Repeated measures ANOVA were used to compare strain measured during the 5 simulated clinical tests across the 3 conditions, and to evaluate the change in strain after contracting the posterior capsule.
Results
There was a statistically significant interaction between tests and conditions for the middle region of the posterior shoulder. In the experimentally contracted condition, strain was greater when GHJ internal rotation was added to humerus flexion than when GHJ internal rotation was added to humerus abduction. There was a statistically significant main effect of tests at the lower region of the posterior shoulder, with internal rotation in abduction and internal rotation in the GHJ resting position demonstrating greater strain than cross-body adduction. The percent change in strain between the baseline and contracted capsule conditions did not reach statistical significance at either region.
Conclusion
Strain on an experimentally contracted posterior GHJ capsule is highest when tested with a combination of GHJ internal rotation and humerus flexion.
Key Terms: Cadaver, Capsule, Contracture, Glenohumeral Joint, Shoulder, Strain
Introduction
Shoulder impingement syndrome (SIS) is the most common musculoskeletal shoulder condition for which people seek medical care.37 Several accepted pathomechanisms for the development of SIS exist, including degenerative changes to the supraspinatus tendon,27 acromion morphology,2,26 scapular or humeral head kinematic alterations,8,12,17,19,20 muscle activation changes,7,39 repetitive overhead activity,18,30 and posterior glenohumeral joint (GHJ) capsule tightness or contracture.1,5 Posterior GHJ capsule tightness is a proposed pathomechanism for both external and internal impingement. External impingement involves compression of the supraspinatus tendon between the humeral head and coracoacromial arch, and the results of inducing experimental posterior capsule tightness in cadavers suggest increased potential for tendon compression through greater anterior and superior humeral head translations during arm elevation.11 Internal impingement is believed to develop most often in overhead athletes when the posterior capsule is theorized to become contracted, shifting the humeral head posterior and superior during the late cocking phase of throwing, and entrapping the articular side of the supraspinatus tendon between the humeral head and glenoid/labrum complex.1,5,6 In throwing athletes with impingement, one consistent clinical finding is a decrease in GHJ internal rotation range of motion,5,15,24,35,36,40 theorized to be the result of posterior GHJ capsule tightness. Based on this loss of GHJ internal rotation in throwing athletes, measuring GHJ internal rotation range of motion at 90° of abduction has traditionally been used to assess posterior GHJ capsule flexibility. However, several more recent clinical tests have been developed to quantify posterior shoulder flexibility.15,25,35,36 Tyler et al36 described a clinical measure of posterior shoulder flexibility using GHJ horizontal adduction at 90° of humeral elevation with the subject sidelying. This measurement was highly correlated with GHJ internal rotation range of motion measured at 90° abduction in a group of throwing athletes.36 Both Laudner et al15 and Myers et al25 later described using a measurement of GHJ horizontal adduction in supine to assess posterior GHJ tightness, which was also highly correlated with GHJ internal rotation range of motion measured at 90° abduction in throwing athletes. Despite the relationship among GHJ horizontal adduction and GHJ internal rotation reported in these studies, the construct validity of the measurements in assessing flexibility of the posterior GHJ capsule has not been established.
A central issue in the lack of validity for measuring the flexibility of the posterior GHJ capsule is that it is not known which tissue from the posterior aspect of the shoulder is limiting the motion. Laudner et al15 discuss this by noting that in addition to measuring motion of the posterior GHJ capsule, their supine measurement also measures the motion of the posterior deltoid, infraspinatus, teres minor, and latissimus dorsi muscles. Other authors note that posterior shoulder measurements are likely influenced by the extensibility of the external rotator cuff muscles,29 and the measurements may also be impacted by a mechanical block on the anterior aspect of the joint.25 So, although these clinical tests were developed on strong clinical experience, reasoning, and judgment, their ability to actually measure flexibility or tightness of the posterior GHJ capsule tissue has not been directly tested.
Because directly measuring tissue behaviors in human subjects is seldom possible, cadavers can be used as a model to examine questions regarding soft tissue response to loads.23 The purpose of this study was to use cadaver shoulder specimens to quantify the strain on selected posterior shoulder tissues using several different clinical test procedures intended to load those tissues. Our hypotheses, based on previous studies,15,25,35,36 were that GHJ horizontal adduction would generate the greatest amount of strain on the posterior shoulder, and that GHJ horizontal adduction would also be most affected by an experimental contraction of the posterior GHJ capsule.
Methods
Specimens
Eight fresh (n=5) or fresh-frozen (n=3) cadaver extremities from 5 individual cadaver specimens (2 F, 3 M) were used for this study (mean age 80.9 yrs). Fresh specimens were stored at temperatures between 34° and 36°F and tested within 48 hours of receiving the donor body. When the laboratory was not immediately available for testing, specimens were frozen to −20°F and thawed 12 hours prior to the experiment. Tissue mechanical properties are not significantly different after a freeze/thaw cycle.38 Each specimen consisted of a scapula and entire upper extremity. Specimens were prepared for testing by removing the skin over the scapula and upper humerus before rigidly securing the scapula to a custom-made wooden frame. The scapula was positioned in anatomic neutral position with the medial border vertical and the plane of the scapula oriented 30° anterior to the frontal plane. This angle was kept consistent by cutting a 30° wedge from the wooden testing post to which the scapula was secured. Data collection was initiated but not completed on 2 other specimens, 1 of which was determined to have undergone a total shoulder arthroplasty, and 1 whose posterior GHJ capsule tore early in the experiment.
Design and Procedures
A repeated measures design was used to compare 5 simulated clinical tests of posterior shoulder tightness across 3 posterior shoulder conditions. The order of presentation of the simulated tests was randomized using a computer generated sequence. With the scapula rigidly secured to the testing frame and unable to rotate, GHJ elevation angles used for the simulated clinical tests were adjusted downward during testing.23 For example, a clinical angle of 90° arm elevation would normally have approximately 60° GHJ rotation and 30° scapula upward rotation, but in the absence of scapula motion the corresponding angle for the experiment was 60° of humeral elevation. By design, the arm elevation angles used for the 5 simulated clinical tests were not precisely controlled during the experiment in order to accurately represent how clinical tests are applied.
The 5 simulated clinical tests and, if applicable, the clinical test positions they attempt to replicate are described and pictured in Table 1. The simulated tests for the experiment include: 1) Cross-Body Adduction, tested at 60° of humerus elevation and selected to approximate the supine and sidelying horizontal adduction clinical measurements; 15,25,35,36 2) 60 ABD, tested at 60° humerus abduction and equivalent to measuring GHJ internal rotation in supine at 90° abduction;15,25,35,36 3) GH Neutral, tested at 40° of scapula plane elevation and, because it approximates the GHJ in its resting position, selected to serve as a baseline test; 4) 60 Flexion, tested at 60° of humerus flexion in the sagittal plane, a position that combines GHJ horizontal adduction and internal rotation; 5) 40 Flexion, tested at 40° of humerus flexion in the sagittal plane and selected because it approximates a modified posterior shoulder tissue stretch position.22
Table 1.
Experimental positions and procedures used to quantify strain on the posterior shoulder tissues. Abbreviations: GH, glenohumeral; IR, internal rotation; ER, external rotation.
| Simulated Clinical Test | Experimental Position and Procedure | Experimental Position | Clinical Equivalent |
|---|---|---|---|
| GH Neutral | 40° Elevation in plane of scapula with neutral GH internal/external rotation; Add GH internal rotation. |  |
Approximates the GH Joint Resting Position |
| 60 ABD | 60° Abduction‡; Add GH internal rotation |  |
Supine IR at 90° Abduction Supine arm elevation in the coronal plane to 90° with the elbow at 90° and the forearm vertical; Measure maximum passive GH IR |
| Cross-body Adduction | 60° Elevation with neutral GH internal/external rotation; Add humerus horizontal adduction |  |
Horizontal Adduction Elevate the arm in the coronal plane to 90° with the elbow flexed 90°; Adduct the humerus in the transverse plane to the end of passive range while maintaining the forearm in the transverse plane. |
| 60 Flexion | 60° Flexion (Sagittal plane); Add GH internal rotation |  |
Similar to the Hawkins-Kennedy Impingement Test Position |
| 40 Flexion | 40° Flexion (Sagittal plane); Add GH internal rotation |  |
Approximates the Modified Sleeper Stretch Position for Posterior Shoulder Tissues |
All simulated clinical tests included 2kg of overpressure to either GH internal rotation or horizontal adduction.
Abduction was operationally defined as 15° anterior to the coronal plane.
All data collection began with the shoulder positioned in GH Neutral before moving the humerus to the simulated clinical test position. When the GH Neutral simulated clinical test was analyzed the humerus was not moved from this starting position. For each simulated clinical test, after the point of passive tissue resistance was reached, a 2 kg manual force was applied perpendicular to the cadaver forearm (creating GHJ internal rotation) or humerus (creating horizontal adduction) to ensure that a controlled amount of load was applied to the posterior shoulder tissues in each simulated test and condition. The point of application of the force was controlled by placing a mark on the forearm 20 cm from the axis of rotation for GHJ internal rotation (represented by the tip of the olecranon process), and a mark on the humerus 20 cm from the axis of rotation for horizontal adduction (an estimate of the center of the humeral head) with a surgical marker. The 2 kg force was ensured by placing a hand-held dynamometer between the testers force application and the cadaver forearm or humerus. The same physical therapist with 16 years orthopedic experience performed 3 repetitions of each simulated test. One repetition consisted of first reaching the end of passive joint movement, then rotating the joint until the 2 kg force was reached and then releasing the force.
To determine an estimate of the amount of force to apply during the simulated clinical tests, 3 experienced clinicians performed the measurements on human subjects with the hand-held dynamometer prior to the experiment. Subjects were positioned in standing for GH Neutral, 60 Flexion, and 40 Flexion, and positioned in supine for 60 ABD and Cross-body Adduction. The mean force used over these 90 measurements (3 clinicians, 3 trials of all 5 clinical tests) was 2.07 kg (SD 0.63 kg, range 1.2–3.9).
The primary dependent variable for this experiment was the amount of strain measured on posterior shoulder tissues during the 5 simulated clinical tests under 3 posterior shoulder conditions. Strain was measured using Microminiature Differential Variable Reluctance Transducers (DVRT) (Microstrain, Inc, Williston, VT; range 3 mm, resolution 1.5 μm, accuracy +1.0%). The first condition (muscle) kept the specimen in its prepared condition with the external rotator cuff muscles and tendons remaining attached at the humerus. In this condition the strain gauges were fixed to the muscles near their tendinous insertions on the humerus. Using the posterior glenoid rim as a guide, the gauges were placed in a position on the muscles that approximated where they would subsequently be placed on the posterior GHJ capsule, resulting in one gauge on the infraspinatus muscle/tendon and another gauge on the teres minor muscle/tendon. The second condition (baseline) exposed the posterior GHJ capsule by first cutting through the midpoints of the infraspinatus and teres minor on the scapula before reflecting the muscles and tendons laterally and carefully removing them from their insertion on the capsule. The third condition (contracted) required the posterior GHJ capsule to be contracted approximately 20 percent. The area of the capsule that was targeted for contraction was from the glenoid rim medially to the humeral head laterally, and between approximately 11:00 superiorly and 6:00 inferiorly using the glenoid as a clock face. A 20% experimental posterior capsule contraction has been shown to alter humeral head translations in cadaver specimens.6 For these last 2 shoulder conditions, the strain gauges were fixed to the capsule in 2 locations (Figure 1). One gauge was in the middle region, defined as the capsule between 9:00 on the glenoid rim and 9:00 on the humeral head, and was oriented horizontally. The second gauge was in the lower region, defined as between 7:30 on the glenoid and 9:00 on the humeral head, and was oriented obliquely. These 2 regions were selected to represent both the capsule stiffness noted to occur in the general population (middle region) and the posterior-inferior capsule stiffness noted to occur in the throwing shoulder (lower region).33,34 The gauges were secured to the muscle or capsule tissue with small barbed projections attached to each gauge. Both gauges were removed while the capsule was being contracted and then replaced on the capsule. To accurately reproduce placement of the strain gauges between conditions, ink marks were used to denote the two 9:00 and the 7:30 positions on the capsule. After the external rotators were removed a surgical marker was used to mark the estimated midpoint (9:00) of the insertion of the posterior capsule on the glenoid labrum and on the humeral head. A third mark was placed on the capsule insertion halfway between the lowest point on the glenoid (6:00) and the 9:00 mark on the glenoid. In all conditions, the strain gauges were applied in their fully shortened position as recommended by the manufacturer (MicroStrain) with the humerus positioned in a dependent position (adducted with no GHJ flexion/extension or internal/external rotation).
FIGURE 1.
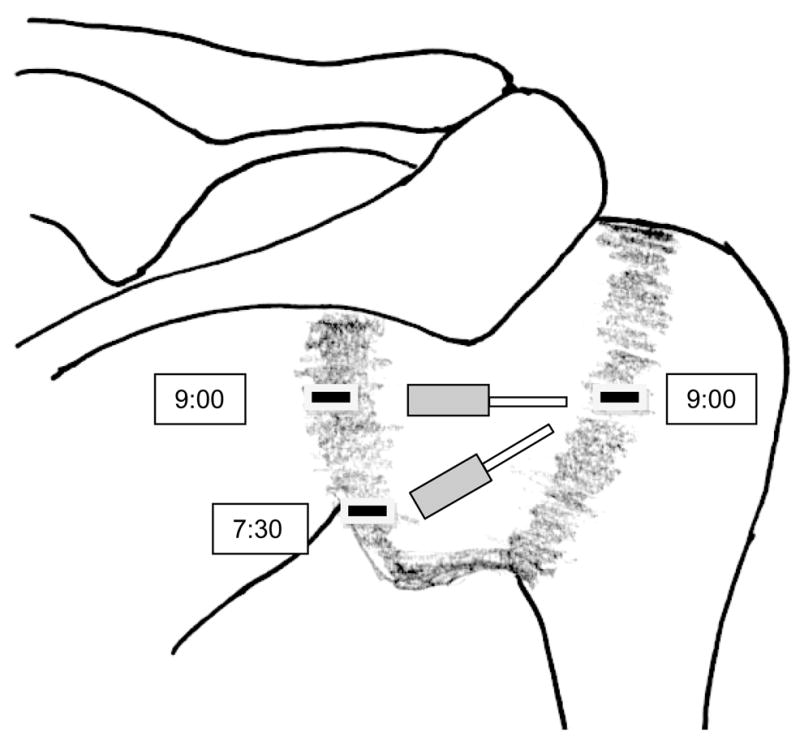
Schematic of posterior glenohumeral joint capsule with location of strain gauges.
In addition to guiding placement of the strain gauges, the two 9:00 marks were used to quantify the amount of capsule contracture. For the contracture procedure, the humerus was placed in the dependent position, and the distance between the two 9:00 marks was measured with a flexible cloth tape measure. Using the flexible tape measure allowed the instrument to follow any contour changes of the capsule and more accurately capture its length. The posterior GHJ capsule was then contracted using radiofrequency energy applied with an Ethicon Mitek VAPR 3 (Mitek, Westwood, MA). The area of posterior capsule contracture described above was exposed to thermal energy to best mimic the type of contracture described in vivo41 and to be consistent with previous studies.32 Thermal energy coagulates and thickens collagen in the capsule tissue32 and while the in vivo capsule thickening process is described as fibrosis, the precise mechanism and microstructural changes associated with fibrosis of the posterior GH capsule remain undescribed.41 Thermal energy was applied to the capsule in horizontal, vertical, and transverse passes until the distance between the two 9 o’clock marks was reduced approximately 20%, as determined by periodically measuring the distance between the 9:00 marks with the humerus in the same dependent position as in the original measurement. The baseline and contracted distances were recorded and the mean change in distance for the sample of 8 specimens was determined.
In addition to quantifying strain, GHJ internal and external rotation range of motion were quantified using a universal 25cm plastic goniometer with the humerus elevated to 60° in the scapular plane (equivalent to 90° scapular plane elevation) in each shoulder condition prior to applying the 5 simulated clinical tests. The axis of rotation of the goniometer was aligned with the long axis of the humerus using the olecranon process as an anatomical landmark. The forearm was used to rotate the humerus until the passive limit of internal or external rotation motion was reached. No added force was applied to rotate the humerus beyond this passive limit. This measurement method is consistent with what is used both clinically and in clinical research.3,4,9,14,22,24 Internal rotation was always measured first and each measurement was made only once per condition per cadaver.
Analysis
Because the arm elevation angles and planes during the 5 simulated clinical tests were not precisely controlled, reliability estimates were calculated for each test. A 1-factor repeated measures ANOVA was used to examine strain over the 3 trials, and intraclass correlation coefficients (ICC 3,1) were calculated from the results. Overall ICCs were calculated by combining all 5 simulated clinical tests across all conditions, and separate ICCs were also calculated for each simulated clinical test under each condition.
For each of the 5 simulated clinical tests in all 3 conditions, the change in length of the strain gauge between the dependent arm position to the end point of the simulated clinical test was calculated for each trial. The mean change in length over the 3 trials was then calculated, divided by the original length to determine strain, and expressed in units of percent change in length by multiplying by 100. Mean percent strain for each simulated clinical test under each condition was then calculated across the sample.
To statistically examine the effect of the posterior shoulder conditions on the simulated clinical tests, 2 separate 2-factor (condition, 3 levels; test, 5 levels) repeated measures ANOVA were run, with mean percent strain at the middle and lower regions of the capsule treated as 2 separate dependent variables. For statistically significant interaction effects, 1-factor (test, 5 levels) repeated measures ANOVA run separately for each condition were planned. Tukey-Kramer post-hoc tests were planned for statistically significant main effects.
To examine the ability of each simulated clinical test to assess a change in flexibility of the posterior GHJ capsule due to the experimental contraction, the difference in mean percent strain was calculated between the baseline and contracted conditions only and analyzed with 2 separate 1-factor (test, 5 levels) repeated measures ANOVA. For GHJ internal and external rotation range of motion, a 1-factor (condition, 3 levels) repeated measures ANOVA examined the effect of condition on range of motion.
Results
In the baseline condition, the mean distance between the two 9 o’clock marks on the posterior capsule was 4.35 cm, while after contracting the capsule the mean distance was 3.53 cm. This reduction of 0.82 cm represents an average experimental contracture of the posterior capsule of 18.9 percent. Reliability testing resulted in overall ICCs (3,1) of 0.90 and 0.89 for the middle and lower regions, respectively. ICCs for individual tests ranged from 0.67 (60 Flexion, Contracted condition, middle region) to 0.90 (60 ABD, Contracted condition, lower region).
For strain measured at the middle region, the ANOVA indicated that there was a statistically significant interaction effect between test and condition (Table 2). Separate 1-factor ANOVA revealed that in the contracted condition, strain in both the 60 Flexion and the 40 Flexion tests was significantly higher (F=5.45; p=0.002) than strain in the 60 ABD test (Figure 2). For strain measured at the lower region, there was not a statistically significant interaction effect between test and condition, but there was a statistically significant main effect of test, with greater strain obtained in the 60 ABD and GH Neutral tests than in the Cross-body Adduction test (Figure 3 and Table 3).
TABLE 2.
Two factor repeated measures ANOVA results.
| Source | df | F-Ratio | p-value | |
|---|---|---|---|---|
| Middle Region | Condition | 2, 14 | 14.84 | <0.001* |
| Test | 4, 28 | 2.77 | 0.047* | |
| Condition × Test | 8, 56 | 2.69 | 0.014* | |
| † Muscle | 4, 28 | 1.00 | 0.426 | |
| † Baseline | 4, 28 | 0.37 | 0.831 | |
| † Contracted | 4, 28 | 5.45 | 0.002* | |
| Lower Region | Condition | 2, 14 | 2.69 | 0.102 |
| Test | 4, 28 | 3.23 | 0.027* | |
| Condition × Test | 8, 56 | 1.66 | 0.129 |
Indicates statistical significance at p<0.05.
Planned 1-way repeated measures ANOVA for each posterior shoulder condition.
FIGURE 2.
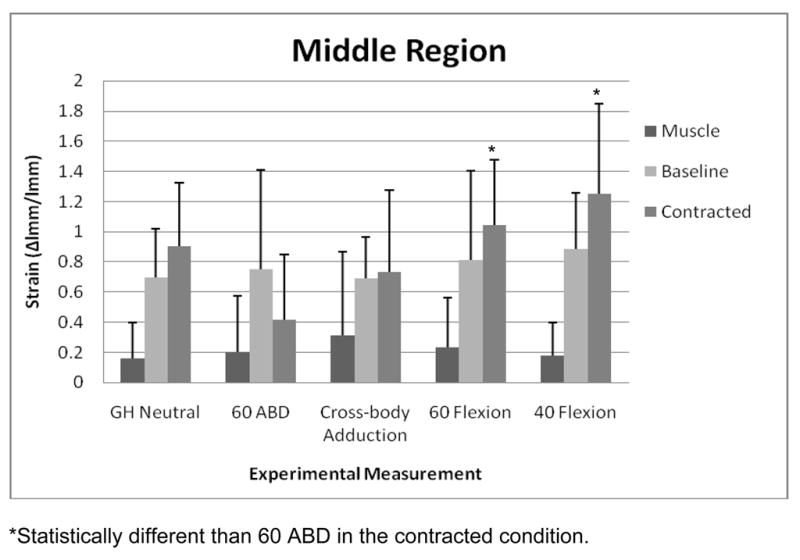
Mean (SD) strain at the middle region of the posterior shoulder during each simulated clinical test across the 3 conditions. Abbreviations: GH, glenohumeral; ABD, abduction.
FIGURE 3.
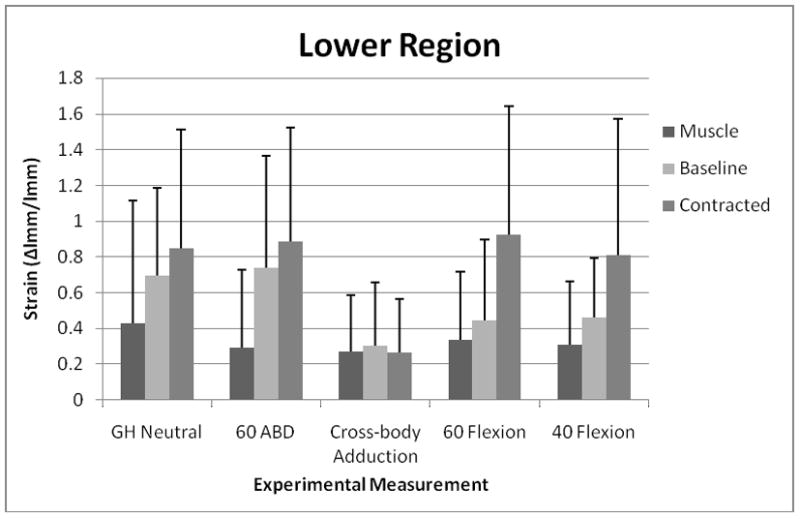
Mean (SD) strain at the lower region of the posterior shoulder during each simulated clinical test across all 3 conditions. Abbreviations: GH, glenohumeral; ABD, abduction.
TABLE 3.
Mean (SD) strain, expressed in percent change in length relative to original length, for the 3 conditions and 5 simulated clinical tests.
| Middle Region | Lower Region | ||
|---|---|---|---|
| Conditions | Simulated Clinical Tests | Mean (SD) | Mean (SD) |
| Muscle# | 22 (35)* | 33 (44) | |
| Baseline# | 77 (45)* | 53 (47) | |
| Contracted# | 87 (55)* | 75 (65) | |
| GH Neutral& | 59 (46) | 66 (62)†† | |
| 60 ABD& | 46 (53)† | 64 (61)†† | |
| Cross-body Adduction& | 58 (49) | 28 (31)†† | |
| 60 Flexion& | 70 (57) | 57 (57) | |
| 40 Flexion& | 77 (61)† | 53 (54) | |
| Muscle | GH Neutral | 16 (24) | 43 (69) |
| 60 ABD | 20 (37) | 29 (44) | |
| Cross-body Adduction | 31 (55) | 27 (32) | |
| 60 Flexion | 23 (33) | 34 (38) | |
| 40 Flexion | 18 (22) | 31 (35) | |
| Baseline | GH Neutral | 70 (32) | 69 (49) |
| 60 ABD | 75 (66) | 74 (62) | |
| Cross-body Adduction | 69 (27) | 30 (35) | |
| 60 Flexion | 81 (59) | 45 (45) | |
| 40 Flexion | 89 (37) | 46 (33) | |
| Contracted | GH Neutral | 91 (42) | 85 (67) |
| 60 ABD | 42 (43)‡ | 89 (63) | |
| Cross-body Adduction | 73 (55) | 27 (30) | |
| 60 Flexion | 105 (43)‡ | 93 (72) | |
| 40 Flexion | 125 (60)‡ | 81 (76) |
Abbreviations: GH, glenohumeral.
Indicates Condition data is collapsed across the 5 simulated clinical tests.
Indicates Test data is collapsed across the 3 conditions.
Baseline and Contracted conditions significantly higher than Muscle condition.
40 Flexion test significantly higher than 60 ABD test.
GH Neutral and 60 ABD tests significantly higher than Cross-body Adduction test.
60 Flexion and 40 Flexion tests significantly higher than 60 ABD test in the contracted condition.
For change in strain, there were no statistically significant differences among tests at either region of the capsule, although at the middle region the main effect of test approached statistical significance (p=0.078). Descriptively, the 60 Flexion and 40 Flexion tests were noted to produce larger change in mean percent strain from baseline to the contracted condition in both the middle and lower regions of the posterior capsule. The 60 ABD test for the middle region and the Cross-body Adduction test for the lower region both resulted in a negative percent change in strain, representing a decrease in strain in the contracted condition compared to baseline (Table 4).
TABLE 4.
Rank of the 5 simulated clinical tests for their ability to detect change in mean strain (%) between contracted and baseline conditions.
| Rank | Measurement | Change (%) | |
|---|---|---|---|
| Middle Region | 1 | 40 Flexion | +37 |
| 2 | 60 Flexion | +23 | |
| 3 | GH Neutral | +21 | |
| 4 | Cross-body Adduction | +04 | |
| 5 | 60 ABD | −33 | |
| Lower Region | 1 | 60 Flexion | +48 |
| 2 | 40 Flexion | +35 | |
| 3 | GH Neutral | +15 | |
| 4 | 60 ABD | +15 | |
| 5 | Cross-body Adduction | −04 |
Positive percent change indicates that there was increased strain produced during the simulated clinical test in the contracted condition.
Statistically, there was a significant condition main effect for GHJ internal rotation range of motion (df=2,14; F=4.72; p=0.03), but not for GHJ external rotation (df=2,14; F=3.28; p=0.07). Tukey-Kramer post-hoc analysis indicated that Baseline internal rotation was greater than Muscle internal rotation by 7.6° (Table 5). Descriptively, mean GHJ internal rotation decreased 5.4° after contracting the posterior capsule, representing a 23.1% reduction in motion compared to the baseline capsule condition.
TABLE 5.
Means (SD) and ranges for glenohumeral joint internal and external rotation range of motion across the 3 posterior shoulder tissue conditions, measured with the humerus elevated to 60° in the scapular plane.
| Internal Rotation | External Rotation | |
|---|---|---|
| Muscle | ||
| Mean | 15.8º (9.2°) | 102.5° (15.1°) |
| Range | 0°– 33° | 68°– 119° |
| Baseline | ||
| Mean | 23.4° (14.2°)* | 105.5° (16.7°) |
| Range | 2°– 49° | 68°– 121° |
| Contracted | ||
| Mean | 18.0° (7.6°) | 107.9° (18.4°) |
| Range | 3°– 25° | 68°– 128° |
Statistically significant difference in internal rotation compared to muscle condition.
Discussion
This experiment quantified strain of posterior shoulder tissues under 5 different simulated clinical tests across 3 unique conditions of the posterior shoulder in cadaver specimens. The hypothesis was that the Cross-body Adduction test would produce the highest strain values, and would be most affected by an experimental contraction of the posterior GHJ capsule. These hypotheses were generated because two different clinical GHJ horizontal (cross-body) adduction measurements have been published and both report strong correlations with supine GHJ internal rotation range of motion,15, 25, 36 a motion restricted by posterior GHJ capsule tightness. In this experiment, strain was not increased significantly when using the Cross-body Adduction test, nor was the test most impacted by the experimental contracture of the posterior GHJ capsule. In addition, the Cross-body Adduction test demonstrated only small changes in strain at both the middle and lower regions of the posterior GHJ capsule (+4% and −4%, respectively). A valid measurement for assessing posterior capsule flexibility should first orient the joint and tissue in a way that when the force is applied, there is optimal loading of the tissue. Because an increase in strain indicates that this optimal loading has occurred during a simulated clinical test, the analysis did not support our hypothesis that the Cross-body Adduction test would generate the greatest strain and be the best of the 5 tests to detect an experimental contracture of the posterior GHJ capsule.
One potential explanation for the lack of support for the Cross-body Adduction test as a posterior capsule flexibility measurement is that the in vivo reliability and validity studies15, 25, 36 were all done on throwing athletes, a group known to develop posterior shoulder tightness along with a gain of GHJ external rotation and loss of GHJ internal rotation range of motion.1,5 However, throwing athletes also develop increased humeral retroversion,28,31 which will also contribute to this shift in GHJ range of motion. Humeral retroversion may have impacted the previous studies by increasing the relative amount of GHJ internal rotation required to position the subject correctly, in turn increasing tension on the posterior GHJ capsule prior to the measurements. Similarly, increased retroversion may orient the humeral head in such a way that it presses into the posterior GHJ capsule more so than a normally retroverted joint, also increasing GHJ capsule tension prior to these clinical measurements. It is likely that the subjects in the previous studies had a combination of posterior GHJ capsule tightness and humeral retroversion and the degree to which each may have impacted the clinical horizontal adduction measurements used in those analyses is unknown. Our cadaver experiment did not quantify or control humeral retroversion. Similarly, throwing athletes can have alterations in scapula positioning at rest or with testing that may impact the assessment of posterior GHJ capsule stiffness through changes in the humerus to scapula relationship.
A throwing shoulder cadaver model has been used in other studies6, 10, 13 but was not used for this experiment as the intent of this study was only to examine the ability of the simulated clinical tests to measure posterior shoulder tissues. Therefore in this experiment, contracting and measuring strain at the lower region of the posterior GHJ capsule served as the method to begin assessing the validity of posterior GHJ capsule tightness measurements in throwing athletes. Without a statistically significant interaction effect, this experiment was not able to statistically support a specific test for the lower region of the capsule. The post-hoc analysis of the statistically significant main effect for test indicated that the GH Neutral and 60 ABD tests produced higher strain than the Cross-body Adduction test. However, because these means are combined across all 3 conditions it may be less specific for discerning posterior GHJ capsule tightness. Although not statistically significant, the highest change in strain between baseline and contracted conditions in the lower region was produced with the 60 Flexion (+48%) and 40 Flexion (+35%) tests, the lowest change was produced with the Cross-body Adduction test (−4%), while the GH Neutral (+15%) and the 60 ABD (+15%) tests produced modest changes in strain. This supports the potential for using some degree of sagittal plane flexion plus GHJ internal rotation to measure changes in lower region posterior GHJ capsule flexibility. Throwing shoulder cadaver models also create anterior capsule laxity and mimic the shift in range of motion and an examination of these simulated clinical tests in a throwing shoulder model may clarify the findings of the present study. It may also be necessary to add increased humeral retroversion to the cadaver model to fully examine the tests applicability to throwing athletes.
For the middle region of the posterior GHJ capsule, the change in strain between baseline and contracted conditions approached statistical significance at p=0.078, and descriptively, it appears that the 60 Flexion, 40 Flexion, and GH Neutral tests were more affected by the experimental contracture than were Cross-Body Adduction and 60 ABD. The primary ANOVA supports this with the 60 Flexion and 40 Flexion tests significantly greater than the 60 ABD test in the contracted condition at the middle region. One obvious issue with using the 60 Flexion test as an assessment for posterior capsule contracture is that it is clinically similar to the Hawkins-Kennedy test, a provocative test for SIS. If the Hawkins-Kennedy test is used to provoke pain as part of the examination for impingement, it may not be a useful or practical test position to also assess posterior GHJ capsule flexibility. Because the 40 Flexion test was also affected by capsule contracture and this position is less likely to provoke impingement pain due to its lower flexion angle, it has the potential to be a better clinical measurement. However, the amount of GHJ internal rotation was not quantified during any of the tests, so the clinical use of either the 60 Flexion or 40 Flexion tests as posterior GHJ capsule flexibility measurements is premature. The relationship between the degree of GHJ posterior capsule contracture and the change in GHJ internal rotation range of motion should be examined.
One concern regarding the clinical measurement of posterior GHJ capsule flexibility or tightness is that the posterior shoulder muscles can also impact the measurement. This experiment addressed this by also measuring strain on the infraspinatus and teres minor during the simulated clinical tests. Observation of the overall mean strains indicates that minimal strain at the infraspinatus, and slightly more strain at the teres minor was produced during the simulated tests. Mean strain in the muscle condition was always lower than the baseline condition. At the middle region/infraspinatus, mean strain produced on the muscle across all tests was significantly less than strain produced by tests in the baseline condition, while at the lower region/teres minor the difference in mean strain between conditions did not reach statistical significance. We cautiously interpret this to mean that passive tension of muscle tissue minimally contributes to the measurement of posterior shoulder tightness and that the test procedures are more likely affecting the posterior GHJ capsule. Our reasons for caution include the inability to measure strain on the muscle and the capsule simultaneously, not altering the stiffness of muscle to examine how this may have affected the tests, and the potential for testing order effects as data were always collected in the muscle condition prior to the baseline condition.
GHJ internal rotation range of motion was influenced by the posterior shoulder conditions, with a statistically significant increase in internal rotation at baseline compared to the muscle condition. The 5.4° loss of GHJ internal rotation after experimental contracture was not statistically significant compared to baseline. This suggests that the passive stiffness of the muscles was high enough to limit passive GHJ range of motion, but not high enough to be significantly influenced by the 2kg force applied during the simulated clinical tests. Based on these GHJ internal rotation changes, it may be beneficial in the future to also experimentally simulate changes in muscle stiffness and examine the effect on both range of motion and the simulated clinical tests. While our reported GHJ internal rotation values are low, they are similar to values reported in other cadavers studies.10,23
The results of this study should be considered in light of several limitations. First, the generalizability of this study on older cadavers to the population that presents with impingement, including throwing athletes, is a concern. Although the consequences of posterior capsule experimental contraction on joint movement and these simulated tests do have applicability to clinical practice, further evaluation of these tests is necessary prior to clinical use. Second, because the simulated clinical test positions/angles were not rigidly controlled, the accuracy of the strain measurements may have been diluted. While this is true, the lack of precise control more accurately reflects clinical practice and likely results in greater within-measurement variability in strain and more difficulty finding statistically significant differences between the simulated tests. Third, strain is only measured in one direction during these simulated tests and the capsule is likely loaded in ways that are not captured with this 1-dimensional method. We suspect that this phenomenon is responsible for the negative strain values, where the strain gauges were fully shortened in the resting position, lengthened moving to GH Neutral, but then shortened again during some tests due to non-linear loading of the capsule. Similarly, the entire posterior capsule was not instrumented for testing, nor did the experimental contracture cover the entire posterior capsule. Fourth, we cannot make any claims about the mechanical or material properties of the posterior capsule as a result of the thermal contraction. We assume that the tissue is less elastic following the contracture because the thermal energy coagulates the collagen in the tissue, but this would need to be confirmed with testing. Fifth, all the simulated clinical tests were conducted in an “upright” position, while some of the clinical equivalent measurements are performed in supine or sidelying. This is potentially important because the effect of gravity on the clinical measurements is changed when the positions differ. The use of a 2kg force during each simulated clinical test was used to help control this limitation and our data indicate that strain changes due to gravity were minimal and were almost exclusively the result of the applied force. Finally, not all clinical measurements stabilize the scapula during their application, while in this experiment the scapula was rigidly fixed during all simulated clinical tests.
Conclusions
Contrary to our hypothesis, the Cross-body Adduction test was not determined to be the best simulated clinical test for producing strain on the posterior GHJ capsule. Rather, the 60 Flexion and 40 Flexion tests produced greater strain on the middle region of the experimentally contracted posterior GHJ capsule. From this experiment, it appears that a combination of GHJ flexion plus GHJ internal rotation optimally produces strain of the posterior GHJ capsule. Further evaluation of the clinical applicability of the 60 Flexion and 40 Flexion tests is warranted. Neither the Cross-body Adduction test nor the 60 ABD test were strongly affected by changes in posterior capsule experimental contracture.
Acknowledgments
Grant Support: This project was funded by The National Institute of Child Health and Human Development, Grant # 1 K01 HD052797-01A1
Contributor Information
John D. Borstad, The Ohio State University, Columbus, OH.
Amitabh Dashottar, The Ohio State University, Columbus, OH.
References
- 1.Bach HG, Goldberg BA. Posterior capsular contracture of the shoulder. J Am Acad Orthop Surg. 2006;14(5):265. doi: 10.5435/00124635-200605000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Bigliani L, Morrison D, April E. The morphology of the acromion and its relationship to rotator cuff tears. Orthop Trans. 1986;10(2):216. [Google Scholar]
- 3.Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: A theoretical and evidence-based perspective. Sports Med. 2008;38(1):17–36. doi: 10.2165/00007256-200838010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Borstad JD, Mathiowetz KM, Minday LE, Prabhu B, Christopherson DE, Ludewig PM. Clinical measurement of posterior shoulder flexibility. Man Ther. 2007;12(4):386–9. doi: 10.1016/j.math.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: Spectrum of pathology part I: Pathoanatomy and biomechanics. Arthroscopy: J of Arthroscopic Rel Surg. 2003;19(4):404–20. doi: 10.1053/jars.2003.50128. [DOI] [PubMed] [Google Scholar]
- 6.Clabbers KM, Kelly JD, Bader D, Eager M, Imhauser C, Siegler S, Moyer RA. Effect of posterior capsule tightness on glenohumeral translation in the late-cocking phase of pitching. J Sport Rehabil. 2007;16(1):41–9. doi: 10.1123/jsr.16.1.41. [DOI] [PubMed] [Google Scholar]
- 7.Cools AM, Witvrouw EE, Declercq GA, Danneels LA, Cambier DC. Scapular muscle recruitment patterns: Trapezius muscle latency with and without impingement symptoms. Am J Sports Med. 2003;31(4):542. doi: 10.1177/03635465030310041101. [DOI] [PubMed] [Google Scholar]
- 8.Deutsch A, Altchek DW, Schwartz E, Otis JC, Warren RF. Radiologic measurement of superior displacement of the humeral head in the impingement syndrome. J Shoulder Elbow Surg. 1996;5(3):186–93. doi: 10.1016/s1058-2746(05)80004-7. [DOI] [PubMed] [Google Scholar]
- 9.Ellenbecker TS, Roetert EP, Piorkowski PA, Schulz DA. Glenohumeral joint internal and external rotation range of motion in elite junior tennis players. J Orthop Sports Phys Ther. 1996;24(6):336–41. doi: 10.2519/jospt.1996.24.6.336. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick MJ, Tibone JE, Grossman M, McGarry MH, Lee TQ. Development of cadaveric models of a thrower’s shoulder. J Shoulder Elbow Surg. 2005;14(1S):49–57. doi: 10.1016/j.jse.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Harryman D, Sidles JA, Clark JM, McQuade KJ, Gibb TD, Matsen FA. Translation of the humeral head on the glenoid with passive glenohumeral motion. J Bone Joint Surg. 1990;72(9):1334–43. [PubMed] [Google Scholar]
- 12.Hebert L, Moffet H, McFadyen B, St-Vincent G. A method of measuring three-dimensional scapular attitudes using the optotrak probing system. Clin Biomech. 2000;15(1):1–8. doi: 10.1016/s0268-0033(99)00032-7. [DOI] [PubMed] [Google Scholar]
- 13.Huffman GR, Tibone JE, McGarry MH, Phipps BM, Lee YS, Lee TQ. Path of glenohumeral articulation throughout the rotational range of motion in a thrower’s shoulder model. Am J Sports Med. 2006;34(10):1662. doi: 10.1177/0363546506287740. [DOI] [PubMed] [Google Scholar]
- 14.Kibler W, Chandler T. Range of motion in junior tennis players participating in an injury risk modification program. J Sci Med Sport. 2003;6(1):51–62. doi: 10.1016/s1440-2440(03)80008-7. [DOI] [PubMed] [Google Scholar]
- 15.Laudner KG, Stanek JM, Meister K. Assessing posterior shoulder contracture: The reliability and validity of measuring glenohumeral joint horizontal adduction. J Athl Train. 2006;41(4):375. [PMC free article] [PubMed] [Google Scholar]
- 16.Laudner KG, Myers JB, Pasquale MR, Bradley JP, Lephart SM. Scapular dysfunction in throwers with pathologic internal impingement. J Orthop Sports Phys Ther. 2006;36(7):485–94. doi: 10.2519/jospt.2006.2146. [DOI] [PubMed] [Google Scholar]
- 17.Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80(3):276. [PubMed] [Google Scholar]
- 18.Ludewig P, Borstad J. Effects of a home exercise programme on shoulder pain and functional status in construction workers. Br Med J. 2003;60(11):841. doi: 10.1136/oem.60.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludewig PM, Cook TM. Translations of the humerus in persons with shoulder impingement symptoms. J Orthop Sports Phys Ther. 2002;32(6):248–59. doi: 10.2519/jospt.2002.32.6.248. [DOI] [PubMed] [Google Scholar]
- 20.Lukasiewicz AC, McClure P, Michener L, Pratt N, Sennett B. Comparison of 3-dimensional scapular position and orientation between subjects with and without shoulder impingement. J Orthop Sports Phys Ther. 1999;29(10):574–83. doi: 10.2519/jospt.1999.29.10.574. [DOI] [PubMed] [Google Scholar]
- 21.Magee DJ. Orthopedic physical assessment. 5. Saunders; St. Louis, MO: 2007. [Google Scholar]
- 22.McClure P, Balaicuis J, Heiland D, Broersma ME, Thorndike CK, Wood A. A randomized controlled comparison of stretching procedures for posterior shoulder tightness. J Orthop Sports Phys Ther. 2007;37(3):108–14. doi: 10.2519/jospt.2007.2337. [DOI] [PubMed] [Google Scholar]
- 23.Muraki T, Aoki M, Uchiyama E, Miyasaka T, Murakami G, Miyamoto S. Strain on the repaired supraspinatus tendon during manual traction and translational glide mobilization on the glenohumeral joint: A cadaveric biomechanics study. Man Ther. 2007;12(3):231–9. doi: 10.1016/j.math.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Myers JB, Laudner KG, Pasquale MR, Bradley JP, Lephart SM. Glenohumeral range of motion deficits and posterior shoulder tightness in throwers with pathologic internal impingement. Am J Sports Med. 2006;34(3):385. doi: 10.1177/0363546505281804. [DOI] [PubMed] [Google Scholar]
- 25.Myers JB, Oyama S, Wassinger CA, Ricci RD, Abt JP, Conley KM, Lephart SM. Reliability, precision, accuracy, and validity of posterior shoulder tightness assessment in overhead athletes. Am J Sports Med. 2007;35(11):1922. doi: 10.1177/0363546507304142. [DOI] [PubMed] [Google Scholar]
- 26.Neer CS. Impingement lesions. Clin Orthop. 1983;173:70. [PubMed] [Google Scholar]
- 27.Nirschl RP. Rotator cuff tendinitis: Basic concepts of pathoetiology. Instr Course Lect. 1989;38:439–45. [PubMed] [Google Scholar]
- 28.Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30(3):347. doi: 10.1177/03635465020300030801. [DOI] [PubMed] [Google Scholar]
- 29.Poser A, Casonato O. Posterior glenohumeral stiffness: Capsular or muscular problem? A case report. Man Ther. 2008;13(2):165–70. doi: 10.1016/j.math.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Punnett L, Fine LJ, Keyserling WM, Herrin GD, Chaffin DB. Shoulder disorders and postural stress in automobile assembly work. Scand J Work Environ Health. 2000;26(4):283–91. doi: 10.5271/sjweh.544. [DOI] [PubMed] [Google Scholar]
- 31.Reagan K, Meister K, Horodyski MB, Werner DW, Carruthers C, Wilk K. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002;30(3):354. doi: 10.1177/03635465020300030901. [DOI] [PubMed] [Google Scholar]
- 32.Selecky MT, Tibone JE, Yang BY, McMahon PJ, Lee TQ. Glenohumeral joint translation after arthroscopic thermal capsuloplasty of the rotator interval. J Shoulder Elbow Surg. 2003;12(2):139–43. doi: 10.1067/mse.2003.26. [DOI] [PubMed] [Google Scholar]
- 33.Tehranzadeh AD, Fronek J, Resnick D. Posterior capsular fibrosis in professional baseball pitchers: Case series of MR arthrographic findings in six patients with glenohumeral internal rotational deficit. Clin Imaging. 2007;31(5):343–8. doi: 10.1016/j.clinimag.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Tuite MJ, Petersen BD, Wise SM, Fine JP, Kaplan LD, Orwin JF. Shoulder MR arthrography of the posterior labrocapsular complex in overhead throwers with pathologic internal impingement and internal rotation deficit. Skeletal Radiol. 2007;36(6):495–502. doi: 10.1007/s00256-007-0278-6. [DOI] [PubMed] [Google Scholar]
- 35.Tyler TF, Nicholas SJ, Roy T, Gleim GW. Quantification of posterior capsule tightness and motion loss in patients with shoulder impingement. Am J Sports Med. 2000;28(5):668. doi: 10.1177/03635465000280050801. [DOI] [PubMed] [Google Scholar]
- 36.Tyler TF, Roy T, Nicholas SJ, Gleim GW. Reliability and validity of a new method of measuring posterior shoulder tightness. J Orthop Sports Phys Ther. 1999;29(5):262–9. doi: 10.2519/jospt.1999.29.5.262. discussion 270–4. [DOI] [PubMed] [Google Scholar]
- 37.Van der Windt D, Koes B, De Jong B, Bouter L. Shoulder disorders in general practice: Incidence, patient characteristics, and management. Br Med J. 1995;54(12):959. doi: 10.1136/ard.54.12.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Ee C, Chasse A, Myers B. Quantifying skeletal muscle properties in cadaveric test specimens: Effects of mechanical loading, postmortem time, and freezer storage. J Biomech Eng. 2000;122:9. doi: 10.1115/1.429621. [DOI] [PubMed] [Google Scholar]
- 39.Wadsworth D, Bullock-Saxton I. Recruitment patterns of the scapular rotator muscles in freestyle swimmers with subacromial impingement. Int J Sports Med. 1997;18(8):618–24. doi: 10.1055/s-2007-972692. [DOI] [PubMed] [Google Scholar]
- 40.Warner JJP, Micheli LJ, Arslanian LE, Kennedy J, Kennedy R. Patterns of flexibility, laxity, and strength in normal shoulders and shoulders with instability and impingement. Am J Sports Med. 1990;18(4):366. doi: 10.1177/036354659001800406. [DOI] [PubMed] [Google Scholar]
- 41.Yoneda M, Nakagawa S, Mizuno N, Fukushima S, Hayashida K, Mae T, Izawa K. Arthroscopic capsular release for painful throwing shoulder with posterior capsular tightness. Arthroscopy: JArthroscopic Rel Surg. 2006;22(7):801.e1–801.e5. doi: 10.1016/j.arthro.2005.12.056. [DOI] [PubMed] [Google Scholar]


