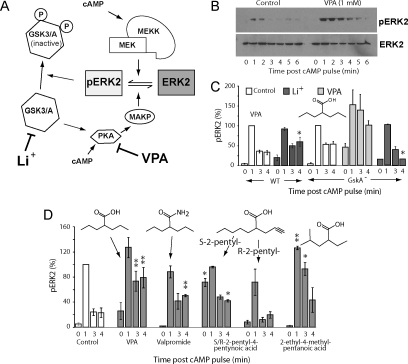
Fig. 2.
MAPK regulation by bipolar disorder treatments in the simple biomedical model Dictyostelium. In both Dictyostelium and mammalian systems, valproic acid (VPA) and lithium (Li+) treatment leads to an increase in activated MAPK, illustrated here for the phosphorylation of the ERK2 (extracellular regulated kinase 2 [ERK2] to form pERK2). (A) Regulation of pERK2 levels is complex, provided by a balance between kinase-dependent phosphorylation (catalysed by MEKK [mitogen-activated protein kinase kinase kinse], and MEK [MAPK kinase kinase]) and by de-phosphorylation (catalysed by MKP [MAP kinase phosphatase] in the PKA [protein kinase A] and GSK3/A [glycogen synthase kinase 3/A] signalling pathway). (B) Treatment of Dictyostelium cells with a single pulse of cyclic AMP (cAMP) gives rise to a transient increase in pERK2 detected by using phosphorylation-specific antibodies. Pre-incubation of Dictyostelium cells with VPA (1 mM, 60 min) increases and elongates the pathway activation by an unknown mechanism. (C) Research in Dictyostelium enables the use of isogenic cell lines containing either ablated or over-expressed genes, providing valuable tools for pharmacological research. For example, we can examine the role of GSKA (homologous to the human GSK3 proteins) in this effect by comparing pERK2 levels in wild-type cells and those lacking GSKA, under control conditions (no added drug) or following Li+ (10 mM, 60 min, dark grey) OR VPA (1 mM, 60 min, light grey) treatment. GSKA ablation increases pERK2 levels, suggesting that it functions as a negative regulator of this pathway, as does pharmacological inhibition with Li+. The lithium-dependent increase in pERK2 is also significantly reduced in the GSKA null, in agreement with a lithium/GSKA-dependent mechanism of action. In contrast, VPA causes a hypersensitive pERK2 increase in this mutant, suggesting VPA functions through a GSKA-independent mechanism. This approach shows an unambiguous role of GSKA in the transient formation of pERK2. * P < 0.05 compared to control. (D) Novel compounds related to VPA can also be analysed in structure–activity relationship studies (SARs), where for example, the amide derivative of VPA (valpromide), enantiomeric compounds (R- and S-2-pentyl-4-pentynoic acid), and altered branch derivatives (2-ethyl-4-methylpentanoic acid) show altered pathway activation. **P < 0.01, *P < 0.05 compared to control.