Abstract
Nociception is a mechanism fundamental to the ability of animals to avoid noxious stimuli capable of causing serious tissue damage. It has been established that in the fruit fly Drosophila melanogaster, the transient receptor potential (TRP) channel encoded by the painless gene (pain) is required for detecting thermal and mechanical noxious stimuli. Little is known, however, about other genetic components that control nociceptive behaviors in Drosophila. The amnesiac gene (amn), which encodes a putative neuropeptide precursor, is important for stabilizing olfactory memory, and is involved in various aspects of other associative and non-associative learning. Previous studies have indicated that amn also regulates ethanol sensitivity and sleep. Here we show that amn plays an additional critical role in nociception. Our data show that amn mutant larvae and adults are significantly less responsive to noxious heat stimuli (> ~ 40 °C) than their wild-type counterparts. The phenotype of amn mutants in thermal nociception, which closely resembles that of pain mutants, was phenocopied in flies expressing amn RNAi, and this phenotype was rescued by the expression of a wild-type amn transgene. Our results provide compelling evidence that amn is a novel genetic component of the mechanism that regulates thermal nociception in Drosophila.
Keywords: Invertebrate, memory gene, painless, neuropeptide, GAL4/UAS, RNA interference
INTRODUCTION
Nociception, the neural process induced by noxious stimuli capable of causing tissue damage, is of vital importance for animal survival. In mammals, nociception is initiated when specialized sensory neurons referred to as nociceptors, including the primary afferent neurons of the dorsal root ganglia, are activated at peripheral terminals by potentially harmful stimuli. This sensory information is then transmitted to spinal cord neurons that project to the cortex through a relay involving the thalamus. This activity ultimately elicits painful sensation and appropriate nociceptive behaviors. Certain ion channels that are present in nociceptors, such as members of the transient receptor potential (TRP) channel superfamily, play an essential role in the detection of noxious stimuli (Numazaki and Tominaga, 2004; Patapoutian et al., 2009). In addition, a wide variety of molecules other than ion channels have been shown to act in concert to positively or negatively modulate information processing in the mammalian nociceptive pathways (Julius and Basbaum, 2001).
The fruit fly Drosophila melanogaster, which is amenable to the detailed genetic analysis of complex biological processes, is an attractive model system in which to unravel the evolutionarily conserved, fundamental mechanisms that underlie the regulation of nociception. It has been shown that nociceptive behaviors in Drosophila, at both the larval and adult stages, can be elicited by activation of one of the TRP channels encoded by the painless gene (pain) (Tracey et al., 2003; Xu et al., 2006; Sokabe et al., 2008). Moreover, a fly homolog of the inflammatory cytokine, tumor necrosis factor-alpha (TNF-α), has been demonstrated to play a role in sensitization of larval nociception (Babcock et al., 2009). However, little is known about other molecular components involved in the regulation of Drosophila nociceptive behavior.
The Drosophila amnesiac gene (amn) is predicted to encode a neuropeptide precursor (Feany and Quinn, 1995; Moore et al., 1998). It was initially identified as a “memory gene”, through a behavioral screen for mutants with abbreviated memory retention (Quinn et al., 1979). Interestingly, although mutants for amn can learn in response to both aversive and appetitive olfactory conditioning, their memory decays abnormally quickly (Quinn et al., 1979; Tempel et al., 1983), indicating that the amn gene product has a critical role in stabilizing olfactory memory. amn mutants also show defects in other types of associative and non-associative learning, including operant visual learning (Gong et al., 1998), courtship conditioning (Sakai et al., 2004) and the acoustic priming of mating behavior (Kyriacou and Hall, 1984). In addition to their defects in learning and memory, amn mutants are abnormally sensitive to ethanol and display aberrant sleep regulation (Moore et al., 1998; Liu et al., 2008). Furthermore, amn appears to have a role in processing the information of innocuous thermal stimuli, as discovered by Hong et al. (2008). These investigators identified amn in a large-scale genetic screen for mediators of “temperature preference behavior”, the fly behavior whereby the animals localize to areas with an ambient temperature close to their desired temperature (24–25 °C for wild-type flies) (Hong et al., 2008).
Here we show that amn mutants exhibit additional defects — in behavioral responses to noxious heat stimuli. The observed phenotype in acute thermal nociception is comparable to that observed in the previously characterized pain mutants. We confirm that the mutation in amn is the cause of defective nociception in amn mutants, demonstrating that their nociceptive phenotype is phenocopied by the application of amn-targeted RNAi and rescued by the expression of wild-type amn transgene. Overall, our results demonstrate that amn is a novel genetic component required for the regulation of thermal nociception in Drosophila, both at the larval and adult stages.
MATERIALS AND METHODS
Fly stocks
Flies were reared at room temperature (22–23 °C) on conventional glucose-yeast-cornmeal agar medium. The wild-type Canton-S (CS) and a white (w) mutant line isogenized to CS were used as controls. pain1 and pain3 stocks were obtained from Dr. Seymour Benzer (California Institute of Technology, deceased). amn1, amnX8 and amn28A stocks were obtained from Dr. Ulrike Heberlein (UCSF), and the amnX8; UAS-amn+ stock was obtained from Dr. Scott Waddell (University of Massachusetts). The daughterless-GAL4 (da-GAL4) and UAS-RNAi stocks for amn were obtained from Dr. Wayne Johnson (University of Iowa) and the Vienna Drosophila RNAi Center (VDRC ID 5606), respectively.
Behavioral assays
Light-driven heat-avoidance assay
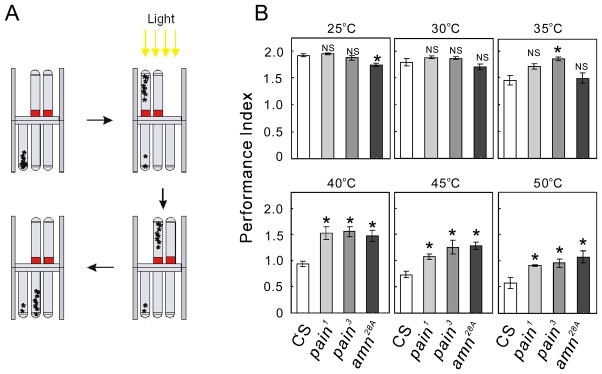
The counter-current apparatus (Benzer, 1967) was modified from the original form and consists of two distal tubes that fit into an aluminum ring, around which is wrapped a band heater powered by a temperature controller (Fig. 1A). The apparatus was placed horizontally, with the tip of the distal tubes facing a source of light. A population of adult flies (approximately 50–70) was placed inside the starting tube (tube 0) and allowed to run toward the light source for 30 seconds, while the aluminum ring was heated to a specific temperature as a heat barrier. The trial was repeated once and the tested flies were sorted into one of three proximal tubes, depending on how many times in two trials they crossed the heat barrier. The performance index (PI) at a particular temperature was calculated as: Σ(i × Ni)/ΣNi, where Ni is the number of flies collected in the ith tube (i=0–2). Wild-type, pain1, pain3 and amn28A flies were tested at 6 experimental temperatures in the range of 25 °C to 50 °C, at increments of 5 °C. Because there was no significant difference in the PI values of males and females (t-test; P > 0.05), except in the case of amn28A at 25 °C (P = 0.026), data for both sexes were combined and analyzed together.
Figure 1.
amn28A mutant flies exhibit reduced responsiveness to high temperatures in the light-driven heat avoidance test. (A) Schematic of the light-driven heat avoidance test, which is carried out using a modified counter-current apparatus equipped with heat bands. In response to a light stimulus, a group of flies (presented as asterisks) are challenged to pass a heat band of the designated temperature. Shown are the steps involved in a single trial. After two consecutive trials, flies were collected into three proximal tubes and the Performance Indices (PIs) were calculated using the indicated formula, where Ni is the number of flies collected in the ith tube. (B) The mean PIs (± SEM) at the designated temperature (25–50 °C) are shown for wild-type flies (CS) as well as for pain1, pain3 and amn28A mutant flies. N=3–5. *P < 0.05, **P < 0.01, ***P < 0.001, for comparison to the value for wild-type flies (one-way ANOVA followed by pairwise multiple comparison with the Holm-Sidak method).
Larval heat-probe assay
Larval nociceptive behavior was analyzed using the method described in (Tracey et al., 2003) with minor modifications. Flies were allowed to lay eggs in vials at room temperature for 6 days, and were transferred to an environmental chamber at 25 °C and 64 % humidity on the seventh day. For nociception assays, foraging 3rd instar larvae were placed in a 35 mm plastic Petri dish. The heat probe consisted of a soldering iron with a copper wire tip. Larvae were touched on abdominal segments (4–6) with a probe heated to 46 °C until a rolling response was elicited or greater than 10 seconds had passed after starting the heat application. Tested larvae were collected and sexed. The experiment was videotaped and response times were evaluated. Response times for male and female larvae did not differ significantly (the Mann-Whitney Rank Sum Test; P > 0.05), and thus data for both sexes were combined and analyzed together.
Adult laser-beam assay
The adult behavior elicited by noxious heat was analyzed using the method described in (Xu et al., 2006), with some modifications. Adult females were collected within 12 hour after eclosion, stored in food vials in groups of 10–15, and maintained for 3–5 days in an environmental chamber at 25 °C and 64 % humidity. Flies were anesthetized with CO2 and the thorax was glued to the pipette tip, dorsal side up. After 30 min recovery time, the flies were allowed to grasp a Kimwipe ball (1~2 mm) and placed in the path of the laser beam 44 mm distant from the laser source, with the head facing away from the laser. The laser used was a PM laser module with a 685 nm, 60 mW diode (LD1216) powered by a 5VDC laser diode control unit (LDCU5) (Power Technology, Inc, Little Rock, AR, USA). The module generated 50 mW output and produced an elliptical laser beam (approximately 3:1 aspect ratio). Under our experimental conditions (distance, focus, and optical power output) the temperature measured using Traceable Digital Thermometer (Control Company, Friendswood, TX, USA) at the position of the fly’s abdomen was about 40 °C. Experiments were video recorded, and the response to noxious heat was quantified as the time from the laser hitting the fly abdomen to the time at which the fly dropped the ball.
All assays for larval and adult nociceptive behaviors were performed between the hours of 10:00 am and 3:00 pm, in an environmental chamber at 25 °C and 64 % humidity. The experimenter was blinded to the genotype of the flies.
RT-PCR assay
Total RNA was extracted from 20 flies using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). One μg of total RNA was converted to cDNA using High Capacity Reverse Transcriptase (Applied Biosystems, Foster City, CA, USA), and one twentieth of the converted cDNA was subjected to PCR, for 30 cycles in the case of amn and 25 cycles in the case of rp49 (internal control). The PCR products were analyzed on agarose gel and signal intensities were normalized to those of flies with no transgene. The primers used were 5′-TGTTTTTATCCGGCTGCTGTGG-3′ and 5′-CCGACCAATCAGAGAGCAGCTTG-3′ for amn, and 5′-GATCGATATGCTAAGCTGTCGC-3′ and 5′-CGACCACGTTACAAGAACTCT-3′ for rp49.
Statistical analysis
Statistical analyses were performed using SigmaStat version 3.11 (Systat Software, Inc, Chicago, IL, USA). A P-value < 0.05 was considered statistically significant. One-way ANOVA, followed by use of the Holm-Sidak method for pairwise multiple comparison, was applied to analyze the data from the light-driven heat-avoidance assay. For the larval and adult nociception assays, medians were statistically compared using the Mann-Whitney Rank Sum Test for single comparisons and the non-parametric Kruskal-Wallis ANOVA, followed by Dunn’s pairwise test for multiple comparisons. The data for nociception assays are presented as box plots, in which the first quartile, the median, and the third quartile are represented by the lower edge, the middle line and the upper edge of each box, respectively. The outliers are shown as dots in the figures. In this study, an outlier was a data point that fell outside of either the lower or upper fence. The lower and upper fences were defined as Q1−1.5 × IQ and Q2+1.5 × IQ, respectively (where Q1, Q2 and IQ are the lower quartile, the upper quartile and the interquartile range, respectively, the intraquartile range being the difference between the 25th and 75th percentiles). The lowest and highest values that are not outliers are shown as whiskers.
RESULTS
amn Mutants Are Less Responsive to Noxious Heat than Wild-type Flies in a Light-driven Heat-avoidance Test
To assess the behavioral responses of adult flies to heat stimuli, we employed a counter-current apparatus equipped with heat bands (Fig. 1A). Using phototaxis to drive the movement of flies, we examined adult flies for their ability to cross the heated band at different temperatures (ranging from 25 °C to 50 °C, at 5 °C increments). The PI was calculated with the formula shown in Fig. 1A. When distributions of flies in tube 0, 1 and 2 are (0, 10, 90%), (20, 30, 50%) and (50, 30, 20%), for example, the corresponding PIs are 1.9, 1.3 and 0.7, respectively. At 25 °C, most wild-type flies passed the heat band twice in two trials (PI = 1.91 ± 0.025). As the temperature of the heat band was increased, the PI value decreased. The wild-type PI at 50 °C (0.58 ± 0.10) was considerably lower than that at 25 °C. At 25 °C and 30 °C, the performances of two pain mutant alleles (pain1 and pain3) were similar to those of wild-type flies (Fig. 1B). In contrast, significant differences were observed between the PIs of wild-type flies (0.94 ± 0.05) and pain mutants (pain1; 1.52 ± 0.12, pain3; 1.55 ± 0.12) at 40 °C (P<0.01). The trend continued at 45 °C and 50 °C (Fig. 1B). These results demonstrated that the light-driven heat-avoidance test involving the modified counter-current apparatus detects the abnormality of pain mutants in thermal nociception, and can be applied to investigate the nociceptive responses of adult flies.
amn28A is a loss-of-function allele of amn. It carries a GAL4-containing P-element in the putative 5′-untranslated region of amn, as well as a second 1.4 kb insertion in the amn open reading frame (ORF) (Moore et al., 1998). Using the modified counter-current apparatus, we found that amn28A displays a behavioral phenotype similar to that of pain mutants. When the temperature of the heat band was below 40 °C, amn28A mutants showed PI values indistinguishable from (at 30 °C and 35 °C), or even lower than (at 25 °C), those of wild-type flies (Fig. 1B). At 40–50 °C, however, a larger proportion of amn28A flies than wild-type flies crossed the heat band. PI values for the amn28A and wild-type flies differed significantly in this temperature range (P < 0.01), whereas those among pain1, pain3 and amn28A mutants at 40–50 °C did not differ significantly (P > 0.05).
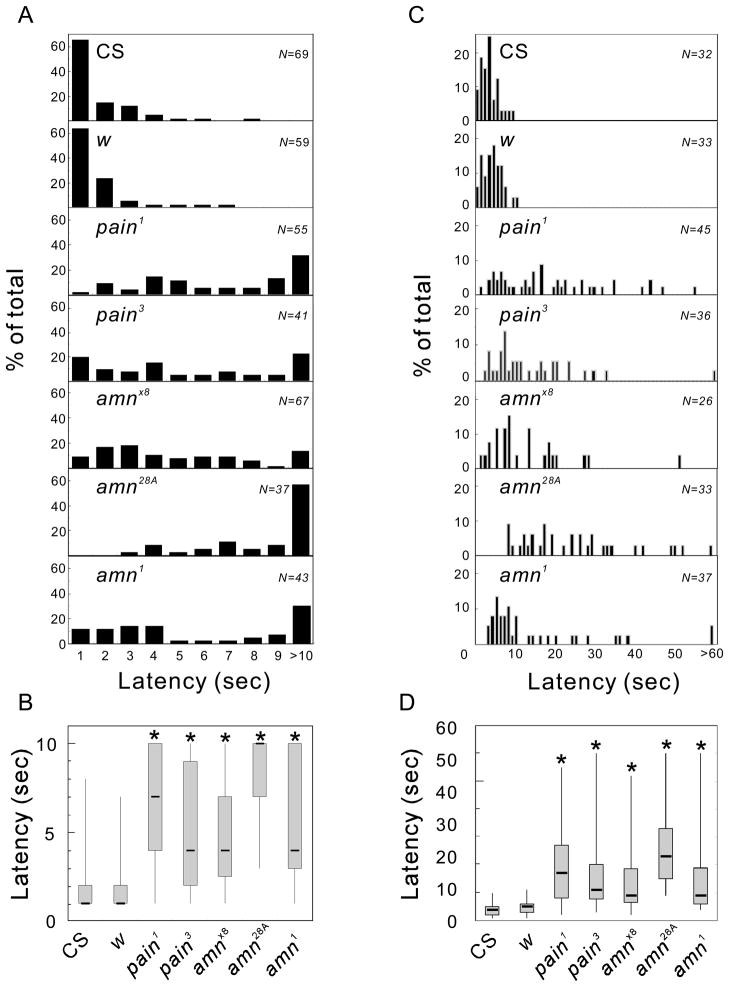
amn Mutant Larvae and Adults Displayed Significantly Longer Latencies in Their Responses to Noxious Heat
The abnormal performance of the amn28A mutant in the light-driven heat-avoidance assay suggested that amn plays an important role in thermal nociception. We tested this possibility by examining three amn mutant alleles (amn1, amnx8 and amn28A) for their behavioral responses to the direct application of noxious heat. amn1 is a chemically induced mutant allele (Quinn et al., 1979). The molecular lesion in the amn1 allele has not been identified, but is thought to lead to a reduction in steady-state levels of amn transcripts (Moore et al., 1998). amnX8 was created by imprecise excision of the P element insertion in amn28A, and lacks the entire amn coding region (Moore et al., 1998).
As in the case of the previous report (Tracey et al., 2003), when wild-type and white (w) mutant larvae were touched with a metal probe heated to 46 °C, they immediately started rolling sideways in a characteristic corkscrew-like motion (rolling behavior). More than 60 % of wild-type and w larvae began this rolling behavior within 1 second of application of the heat stimulus (Fig 2A). Consistent with the results described previously in (Tracey et al., 2003) (Fig. 2A, B), pain1 and pain3 larvae showed a delay in this behavioral response, and like the pain mutant larvae, amn1, amnx8 and amn28A larvae all displayed a significant delay in their responses to noxious heat in comparison to the control flies (P < 0.05). The median response latencies were 3, 10 and 3 seconds for amnx8, amn28A and amn1, respectively (Fig. 2B). The greatest delay was observed for amn28A larvae (Fig. 2A, B); approximately 60 % failed to display rolling behavior even after 10 seconds.
Figure 2.
amn mutants display a longer latency for behavioral responses to directly applied noxious heat. Distributions of response latency in larvae (A) and adult flies (C) are presented as percentage of total number of flies (N). The same data sets are presented as box plots, for larvae (B) and adult flies (D), with the median values indicated by solid horizontal bars and the ranges between the lower and upper quartiles are shown as gray boxes. *P < 0.05, for comparison to values for the wild-type (CS) flies (Krustal-Wallis ANOVA, followed by Dunn’s pairwise test for multiple comparisons).
amn mutant adults also displayed an abnormally long latency in their jump response to noxious heat applied to the abdomen with an infrared laser beam (Fig. 2C, D). In this assay, 100 % of wild-type and 97 % of w mutant adult flies responded to the heat stimulus within 10 seconds, with median response times of 4 and 5 seconds, respectively. As reported previously (Xu et al., 2006), pain mutant adults displayed prolonged latency to the noxious heat stimulus (Fig. 2C, D); in our assay, the median response time for pain1 and pain3 adults were 17 and 11 seconds, respectively. Consistent with the results obtained from larvae, all three amn mutant alleles showed a significantly longer response time than the controls (P < 0.05). The median latencies were 9, 23 and 9 seconds for amnx8, amn28A and amn1 (Fig. 2C, D).
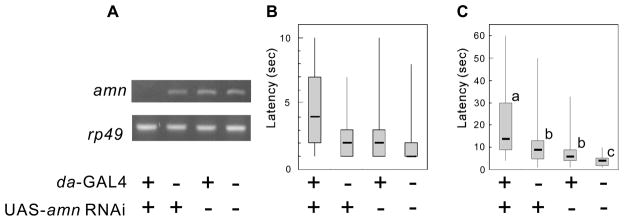
The “pain-like” Thermal Nociceptive Defect in amn Mutants is Phenocopied by Application of amn RNAi and Rescued by Application of a Wild-type amn Transgene
To confirm that the pain-like nociceptive phenotype observed in amn mutants is due to the loss of amn function, we used an amn-targeted RNAi transgene and examined the effect of targeted amn knockdown on nociceptive behaviors. RT-PCR analysis revealed that when the UAS-amn RNAi transgene was expressed ubiquitously using the da-GAL4 driver, the levels of amn transcripts were drastically reduced compared to those in the controls (Fig 3A). Consistent with the results with amn mutants, we found that this RNAi-induced reduction in amn transcripts leads to significantly longer latency in the behavioral responses to a noxious heat stimulus than in controls (P < 0.05), in larvae as well as in adults. The median latency for larvae carrying both the da-GAL4 and UAS-amn RNAi transgenes was 4 seconds, while that for larvae carrying only one of the transgenes (UAS-amn RNAi or da-GAL4) was 2 seconds (Fig 3B, P < 0.05). Likewise, the median response latency for adult flies carrying both da-GAL4 and UAS-amn RNAi was 14 seconds, which differed significantly from the latency for flies carrying only UAS-amn RNAi (9 seconds) or da-GAL4 (6 seconds) (Fig 3C, P < 0.05).
Figure 3.
RNAi-mediated down-regulation of amn causes defects in thermal nociception. (A) RT-PCR analyses revealing the transcript levels of amn in flies carrying da-GAL4 and UAS-amn RNAi transgenes. A representative result of the RT-PCR analysis (top). Ratios of signal intensity of samples (bottom). Average ± standard deviation (STD), N=3. The presence or absence of a transgene is indicated by + or −. The amn transcript levels were decreased in flies carrying both da-GAL4 and UAS-amn RNAi. Latencies of the responses to the noxious heat stimulus are shown as box plots, for both larvae (B) and adult flies (C). The response latency in animals carrying both da-GAL4 and UAS-amn RNAi transgenes was significantly longer than for any other flies. *P < 0.05 (Krustal-Wallis ANOVA, followed by Dunn’s pairwise test for multiple comparisons).
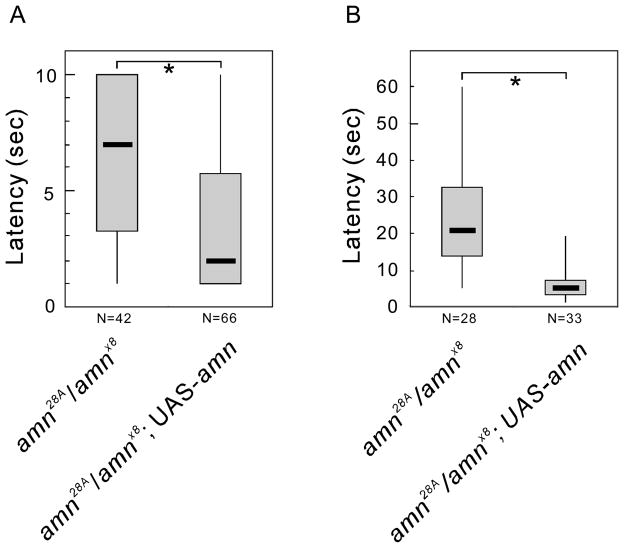
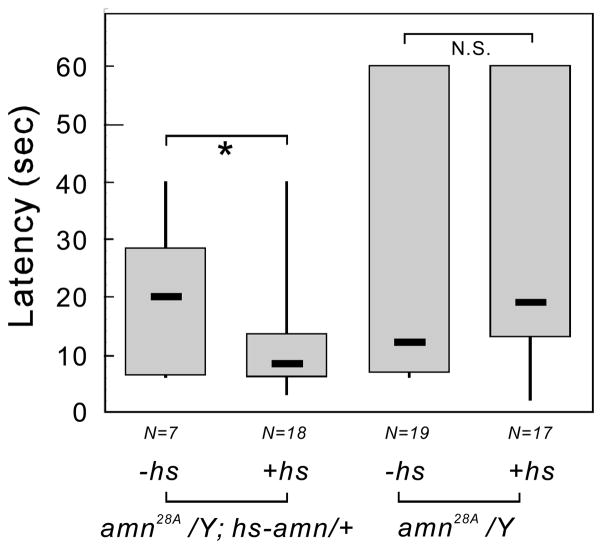
To further confirm that disruption of the amn gene is responsible for the nociceptive phenotype of amn mutants, we carried out rescue experiments using the UAS-amn+ transgene. The GAL4 activity associated with the amn28A mutant is known to direct widespread expression of UAS-reporter genes in the nervous system, both during development and in adulthood (DeZazzo et al., 1999). Moreover, it was previously shown that the expression of a UAS-amn+ transgene in amn28A GAL4 flies results in complete rescue of the amn-associated memory defects (DeZazzo et al., 1999). amnX8, which was created by imprecise P element excision from amn28A, retains the GAL4 activity (Caldwell et al., 2005). We thus were able to cross amn28A females to amnX8 males, with or without the UAS-amn+ transgene, and to test the female progeny (amn28A/amnX8; +/+ or amn28A/amnX8; UAS-amn+/+) for thermal nociception. As had been the case for the memory phenotype (DeZazzo et al., 1999), the amn28A and amnX8 alleles failed to complement each other with respect to thermal nociception in the absence of the amn+ transgene; amn28A/amnX8 females, both larvae and adults, displayed a prolonged latency in their responses to a noxious heat stimulus (Fig. 4). However, when the UAS-amn+ transgene was expressed in these amn28A/amnX8 females using the GAL4 activity associated with the amn28A and amnX8 alleles, the latency in the larval response to heat was drastically reduced, from a median latency of 7 seconds to 2 seconds (Fig. 4A; P < 0.001). The delayed response of amn28A/amnX8 adults to noxious heat was also significantly rescued by the presence of the UAS-amn+ transgene; the median response times to heat were 20.5 seconds and 5 seconds in the absence and presence of UAS-amn+, respectively (Fig. 4B, P < 0.001).
Figure 4.
A wild-type amn+ gene whose expression is driven by amn28A and amnX8 GAL4 activities rescues the thermal nociception defect in amn mutants. Nociception assays were performed for animals trans-heterozygous for amn28A and amnX8, with or without the UAS-amn+ transgene. Latencies of the responses to a noxious heat stimulus are shown as box plots, for larvae (A) and adult flies (B). The amn28A/amnX8; UAS-amn+/+ flies displayed response latencies that were reduced in comparison to those of amn28A/amnX8 flies. ***P < 0.001 (Mann-Whitney Rank Sum Test).
DISCUSSION
amn Regulates Behavioral Responses to Noxious Heat Stimuli
We found that the responsiveness of Drosophila to noxious heat stimuli is significantly impaired in three different amn mutants, amn1, amn28A and amnX8. The amn gene is nested within the intron of another gene CG32529, whose biological function is unknown (Flybase, http://flybase.org/). Because the molecular lesion in amn1 has not been identified and both the amn28A and amnX8 mutations are characterized by large perturbations in the amn locus (Moore et al., 1998), multiple transcripts including those for CG32529 could be affected by these mutations. Therefore, it was possible that the impaired thermal nociception observed in the amn mutants is due to disruption of gene(s) other than amn. However, the results obtained from our RNAi-induced amn knockdown experiment, as well as those from the rescue experiment, provide convincing evidence that the amn gene is indeed involved in the regulation of nociceptive behaviors in both larvae and adults.
Previous studies have shown that there is no significant difference between wild-type and amn mutant flies with respect to their reactivity to a noxious electric shock (DeZazzo et al., 1999). This raises the possibility that the nociception defect in amn mutants is modality-specific and occurs for a limited range of noxious stimuli. Further investigation will be required to determine if amn plays roles in behavioral responses to noxious stimuli other than high temperature.
Previous studies have also shown that amn mutants have defects in both olfactory memory retention and ethanol tolerance (Quinn et al., 1979; Moore et al., 1998). It should be pointed out that the defects in these functions reflect distinct anatomical requirements for amn. In the case of olfactory memory, the mushroom bodies (MBs) are required, and transgenic expression of the amn gene in the Dorsal Paired Medial (DPM) neurons (which ramify to innervate sites throughout the adult MBs) restores odor memory performance to amn mutants (Waddell et al., 2000; Keene et al., 2006). These observations suggest that the predicted amn product is released from DPM neurons and acts through the MBs to stabilize olfactory memory. The sensitivity of adult flies to ethanol, in contrast, is not affected by complete ablation of the MBs, which indicates that the MBs are dispensable for amn-mediated ethanol tolerance (Moore et al., 1998). Likewise, a previous report demonstrated that adult flies with defective MBs display normal thermal nociception (Xu et al., 2003). Therefore, the MBs may be dispensable for amn-mediated regulation of thermal nociception, as appears to be the case for the amn-mediated ethanol tolerance. Interestingly, Hong et al. have shown that the MBs are essential for the fly behavior that determines the desired ambient temperature, a process in which amn has been suggested to play a role (Hong et al., 2008). It is thus likely that amn products regulate two different amn-mediated behaviors — thermal nociception and thermotaxis — by functioning in different cell types. Improving our understanding of possible roles for amn in behavioral response to noxious heat, it will be critical to determine which cells require the amn signal that leads to normal thermal nociception.
Roles of the Putative amn Gene Products in Thermal Nociception
The DNA sequence of the amn locus predicts an ORF of 540 bp, which would be translated into a protein 180-amino acids in length (Feany and Quinn, 1995; Moore et al., 1998). The predicted amn product contains a putative signal sequence and potentially generates three peptides following predicted post-translational processing at two potential cleavage sites. Two of the deduced peptides show weak sequence similarities to mammalian pituitary-adenylyl-cyclase-activating peptide (PACAP) and growth hormone releasing hormone (GHRH). The third predicted peptide does not display any significant similarity to known proteins at the primary sequence level. Although our study demonstrates the significance of the amn gene in thermal nociception, it remains to be determined which potential amn peptides, if any, are relevant to behavioral responses to noxious heat. A similar uncertainty exists for other amn functions, including memory retention, ethanol tolerance and sleep regulation. Rescue experiments using amn transgenes in which the regions corresponding to three predicted amn peptides are separately modified would provide important information regarding this issue.
amn mutants are not completely insensitive to noxious stimuli. As shown in Figure 2, appreciable fractions of amn mutant larvae displayed characteristic nociceptive behavior (i.e., rolling behavior) in response to a 46 °C stimulus, although initiation of the behavior was significantly delayed in amn mutants relative to their controls. We also observed that in amn larvae a 53 °C stimulus elicited a rapid response similar to that in control larvae (data not shown). Furthermore, heat has been effectively used as an negatively reinforcing stimulus in an adult operant visual learning paradigm for amn mutants (Gong et al., 1998), although there is the possibility that decreased thermal sensitivity of the mutants partially contributes to their reported defects in the thermally-reinforced learning. These observations suggest that amn plays modulatory, rather than indispensable, roles in thermal nociception. A recent study has revealed that a tumor necrosis factor (TNF) homolog, Eiger, is involved in pain modulation in Drosophila larvae. Eiger is released from epidermal cells damaged by UV radiation, and sensitizes larval nociceptive behavior through the TNF receptor, Wengen, which is expressed on nociceptive sensory neurons (Babcock et al., 2009). Thus, future studies should explore possible interactions between amn products and components of TNF signaling in Drosophila nociceptive pathways.
Various peptides have been shown to regulate nociceptive behaviors in mammals. PACAP knockout mice display neither inflammatory pain in response to intraplantar injection of inflammation-inducing agents, nor neuropathic pain in response to transection of the L5 spinal nerve transection, although they retain normal nociceptive responses (Mabuchi et al., 2004). This indicates that mammalian PACAP is involved in pain modulation. Mammalian PACAP and GHRH have been shown to stimulate adenylate cyclase through G-protein coupled receptors, and to subsequently increase the cAMP levels in target cells. The Drosophila amn gene product, which has sequence similarity to mammalian PACAP, has also been suggested to activate the cAMP signaling pathway. For example, amn mutations can suppress the female sterility of mutants in which the dunce gene, which encodes cAMP phosphodiesterase (cAMP-hydrolyzing enzyme), is defective (Feany and Quinn, 1995). In addition, increased ethanol sensitivity in amn mutants is likely due to their failure to increase cAMP levels, because mutants for the adenylyl cyclase (cAMP-synthesizing enzyme)-encoding rutabaga gene display a similar ethanol-sensitive phenotype (Moore et al., 1998). Furthermore, the cAMP pathway has been implicated in determining the desired ambient temperature (Hong et al., 2008). Although such temperature preference behaviors and thermal nociceptive behaviors are likely controlled by distinct anatomical regions in the brain, common intracellular mechanisms could be involved in their regulation. It will thus be important to determine if genes involved in cAMP signaling also play a significant role in the regulation of thermal nociception in Drosophila. Given the power of Drosophila genetics and the relative simplicity of the nervous system in this organism, study of the amn-mediated mechanisms in this model should greatly facilitate the process of unraveling how these mechanisms regulate thermal nociception at the molecular, cellular and neural-circuit levels.
Figure 5.
Acknowledgments
We thank Drs. Seymour Benzer, Ulrike Heberlein, Scott Waddell and Wayne Johnson for kindly providing fly strains. This study was supported by NIH grants to T. K. (R03MH078271, R21DA023005 and R01MH062684).
References
- Babcock DT, Landry C, Galko MJ. Cytokine signaling mediates UV-induced nociceptive sensitization in Drosophila larvae. Curr Biol. 2009;19:799–806. doi: 10.1016/j.cub.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proceedings of the National Academy of Sciences of the United States of America. 1967;58:1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell PE, Walkiewicz M, Stern M. Ras activity in the Drosophila prothoracic gland regulates body size and developmental rate via ecdysone release. Curr Biol. 2005;15:1785–1795. doi: 10.1016/j.cub.2005.09.011. [DOI] [PubMed] [Google Scholar]
- DeZazzo J, Xia S, Christensen J, Velinzon K, Tully T. Developmental expression of an amn(+) transgene rescues the mutant memory defect of amnesiac adults. J Neurosci. 1999;19:8740–8746. doi: 10.1523/JNEUROSCI.19-20-08740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Quinn WG. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science. 1995;268:869–873. doi: 10.1126/science.7754370. [DOI] [PubMed] [Google Scholar]
- Gong Z, Xia S, Liu L, Feng C, Guo A. Operant visual learning and memory in Drosophila mutants dunce, amnesiac and radish. Journal of insect physiology. 1998;44:1149–1158. doi: 10.1016/s0022-1910(98)00076-6. [DOI] [PubMed] [Google Scholar]
- Hong ST, Bang S, Hyun S, Kang J, Jeong K, Paik D, Chung J, Kim J. cAMP signalling in mushroom bodies modulates temperature preference behaviour in Drosophila. Nature. 2008;454:771–775. doi: 10.1038/nature07090. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Keene AC, Krashes MJ, Leung B, Bernard JA, Waddell S. Drosophila dorsal paired medial neurons provide a general mechanism for memory consolidation. Curr Biol. 2006;16:1524–1530. doi: 10.1016/j.cub.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Kyriacou CP, Hall JC. Learning and memory mutations impair acoustic priming of mating behaviour in Drosophila. Nature. 1984;308:62–65. doi: 10.1038/308062a0. [DOI] [PubMed] [Google Scholar]
- Liu W, Guo F, Lu B, Guo A. amnesiac regulates sleep onset and maintenance in Drosophila melanogaster. Biochemical and biophysical research communications. 2008;372:798–803. doi: 10.1016/j.bbrc.2008.05.119. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Shintani N, Matsumura S, Okuda-Ashitaka E, Hashimoto H, Muratani T, Minami T, Baba A, Ito S. Pituitary adenylate cyclase-activating polypeptide is required for the development of spinal sensitization and induction of neuropathic pain. J Neurosci. 2004;24:7283–7291. doi: 10.1523/JNEUROSCI.0983-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MS, DeZazzo J, Luk AY, Tully T, Singh CM, Heberlein U. Ethanol intoxication in Drosophila: Genetic and pharmacological evidence for regulation by the cAMP signaling pathway. Cell. 1998;93:997–1007. doi: 10.1016/s0092-8674(00)81205-2. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga M. Nociception and TRP Channels. Current drug targets. 2004;3:479–485. doi: 10.2174/1568007043336789. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn WG, Sziber PP, Booker R. The Drosophila memory mutant amnesiac. Nature. 1979;277:212–214. doi: 10.1038/277212a0. [DOI] [PubMed] [Google Scholar]
- Sakai T, Tamura T, Kitamoto T, Kidokoro Y. A clock gene, period, plays a key role in long-term memory formation in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16058–16063. doi: 10.1073/pnas.0401472101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. Drosophila painless is a Ca2+-requiring channel activated by noxious heat. J Neurosci. 2008;28:9929–9938. doi: 10.1523/JNEUROSCI.2757-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell. 2000;103:805–813. doi: 10.1016/s0092-8674(00)00183-5. [DOI] [PubMed] [Google Scholar]
- Xu J, Culman J, Blume A, Brecht S, Gohlke P. Chronic treatment with a low dose of lithium protects the brain against ischemic injury by reducing apoptotic death. Stroke. 2003;34:1287–1292. doi: 10.1161/01.STR.0000066308.25088.64. [DOI] [PubMed] [Google Scholar]
- Xu SY, Cang CL, Liu XF, Peng YQ, Ye YZ, Zhao ZQ, Guo AK. Thermal nociception in adult Drosophila: behavioral characterization and the role of the painless gene. Genes Brain Behav. 2006;5:602–613. doi: 10.1111/j.1601-183X.2006.00213.x. [DOI] [PubMed] [Google Scholar]