Table 2.
Net contribution of polarization to peptide adsorption on even Au surfaces 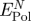 in comparison to the net contribution of epitaxial interaction
in comparison to the net contribution of epitaxial interaction 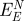 . The main contributions are the polarization energy per peptide
. The main contributions are the polarization energy per peptide 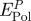 and the loss in polarization energy by replaced surface-bound water
and the loss in polarization energy by replaced surface-bound water 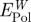 .
.
| surface | peptide | net attraction by polarizationa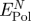 (kJ mol−1) (kJ mol−1) |
net attraction by epitaxyb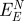 (kJ mol−1) (kJ mol−1) |
contributions to net attraction by polarization |
||
|---|---|---|---|---|---|---|
peptide 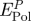 (kJ mol−1) (kJ mol−1) |
replaced surface-bound waterc |
|||||
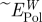 (kJ mol−1) (kJ mol−1) |
no. | |||||
| Au {1 1 1} | A3 | −28 ± 4 [−6 ± 16] | −260 ± 20 | −80 | +74 | 31 |
| Flg-Na3 | −12 ± 4 [−10 ± 16] | −260 ± 20 | −82 | +72 | 30 | |
| Au {1 0 0} | A3 | −16 ± 12 [+10 ± 16] | −38 ± 20 | −23 | +33 | 15 |
| Flg-Na3 | −80 ± 20 [−69 ± 8] | 0 ± 20 | −77 | +9 | 4 | |
aExact values from the difference in polarization energy of the peptide solution relative to a pure aqueous interface for an image plane located at the jellium edge (figure 4). The values in square brackets are simplified additive estimates 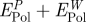 .
.
bFrom Heinz et al. [12]. Using the more accurate CHARMM-METAL force field, lower epitaxial adsorption energies of −160 kJ mol−1 (A3) and −80 kJ mol−1 (Flg-Na3) on {1 1 1} surfaces were reported [15].
cEstimates from an average number count of replaced surface-bound water molecules.
