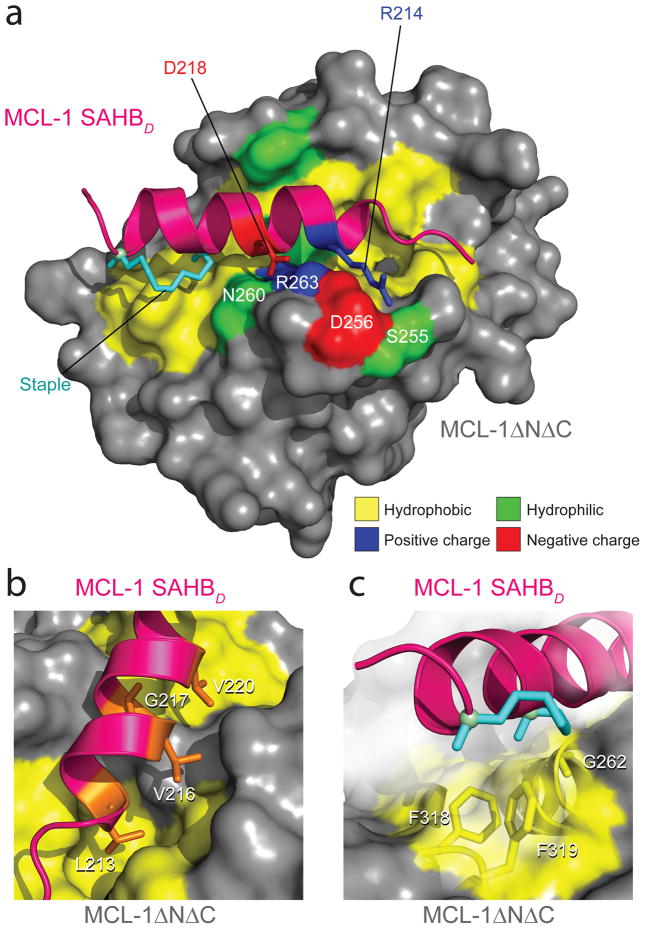
Figure 3.
Crystal structure of the MCL-1 SAHBD/MCL-1ΔNΔC complex. (a) MCL-1 SAHBD engages MCL-1ΔNΔC at the canonical BH3 binding groove of anti-apoptotic proteins, as determined by x-ray crystallography at 2.32-Å resolution (PDB 3MK8). Hydrophobic interactions at the binding interface are reinforced by a complementary polar interaction network that involves MCL-1 SAHBD residues R214 and D218 and MCL-1ΔNΔC residues S255, D256, N260, and R263. The side chains of hydrophobic, positively charged, negatively charged and hydrophilic residues are colored yellow, blue, red and green, respectively. (b) The core BH3 residues L213, V216, G217 and V220 of MCL-1 SAHBD make direct contact with a hydrophobic cleft at the surface of MCL- 1ΔNΔC. (c) The hydrocarbon staple, bearing an olefin in the cis conformation, contributes additional hydrophobic contacts at the perimeter of the core interaction site.