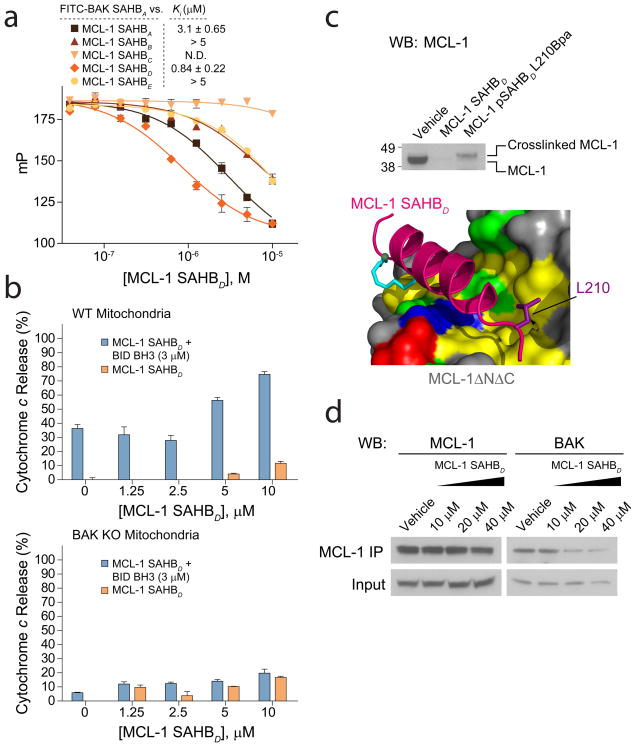
Figure 4.
MCL-1 SAHBD dissociates the inhibitory MCL-1/BAK complex in vitro and in situ, and sensitizes BAK-dependent mitochondrial cytochrome c release. (a) MCL-1 SAHBs effectively prevent sequestration of the BAK BH3 helix by MCL-1ΔNΔC, as demonstrated by competition FPA. N.D., no detected displacement. (b) MCL-1 SAHBD dose-responsively sensitized BID BH3-induced and BAK-dependent mitochondrial apoptosis, as measured by cytochrome c release assay performed on wild type and Bak−/− mitochondria. (c) An OPM2 multiple myeloma cellular lysate was incubated with the indicated biotinylated MCL-1 SAHBD constructs in the presence of ultraviolet light, followed by streptavidin-based purification, stringent washing to remove non-covalent binders, elution, and MCL-1 western analysis. The photoreactive MCL-1 pSAHBD, generated by replacing L210 with a benzophenone-bearing non-natural amino acid (Bpa), directly crosslinked to native MCL-1 within the cellular lysate, whereas no covalent crosslinking was observed for MCL-1 SAHBD, which lacked the photoreactive benzophenone moiety. (d) The native interaction between BAK and MCL-1 was dose-responsively disrupted by treatment of OPM2 cells with MCL-1 SAHBD, as assessed by MCL-1 immunoprecipitation and BAK western analysis. Binding and cytochrome c release data are mean and s.d. for experiments performed in at least triplicate. Vehicle, deionized water.