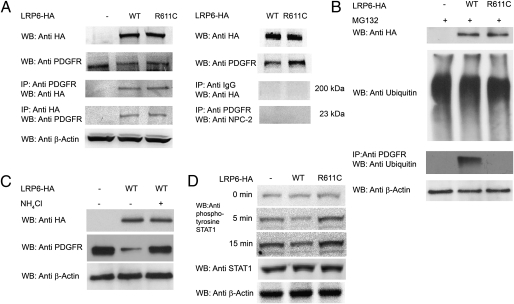
Fig. 5.
LRP6WT and LRP6R611C form complexes with PDGFR-β. (A) Proteins from cell lysates were immunoprecipitated with either anti-HA or anti–PDGFR-β antibodies followed by Western blotting with either anti–PDGFR-β or anti-HA antibodies, respectively. LRP6WT and LRP6R611C but not IgG or NPC2 (used as control) coimmunoprecipitated with PDGFR-β. (B) LRP6WT ubiquitinates PDGFR-β. Ubiquitination of PDGFR-β following MG132 treatment in cells overexpressing LRP6R611C or LRP6WT and in cells infected with empty vector was compared. LRP6WT but not LRP6R611C ubiquitinates PDGFR-β. (C) Lysosomal degradation of PDGFR-β. The lysosomal inhibitor NH4Cl rescues reduced expression of PDGFR-β (P = 0.001). (D) LRP6WT reduces and LRP6R611C increases STAT1 tyrosine phosphorylation. After PDGF stimulation, tyrosine phosphorylation of STAT1 is significantly higher in cells expressing LRP6R611C (P < 0.001) and is significantly lower in cells expressing LRP6WT (P < 0.001) than in cells transfected with empty plasmid.