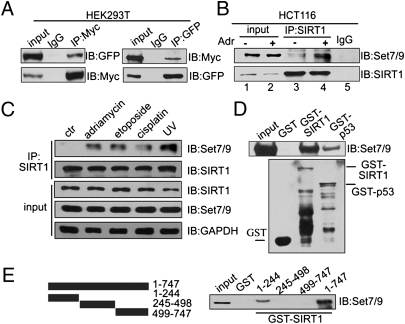
Fig. 2.
SIRT1 interacts with Set7/9 in vitro and in vivo. (A) Expression plasmids for GFP-Set7/9 and Myc-SIRT1 were cotransfected into HEK293T cells and, after 24 h, proteins were extracted and immunoprecipitated by using anti-Myc or anti-GFP, respectively. Western blotting was performed with antibodies as indicated. (B) HCT116 (p53+/+) cells were treated with 1 μM Adr for 6 h, and proteins were extracted for co-IP with anti-SIRT1 and probed with anti-Set7/9. The drug-treated sample was used for the IgG pull-down. (C) HCT116 (p53+/+) cells were treated with 1 μM Adr for 6 h, 20 μM etoposide for 12 h, 10 μM cisplatin for 6 h, or UV-C (60 J/m2). Proteins were extracted for co-IP with anti-SIRT1 and probed with anti-Set7/9. (D) His-Set7/9 protein (cloned into pET28 and expressed in bacteria) was purified in vitro and incubated with GST, GST-SIRT1, or GST-p53 fusion protein (purified from bacteria by using vector pGEX-4T3). Western blotting with anti-Set7/9 was performed to detect the interaction of SIRT1 and Set7/9 (Upper). GST, GST-SIRT1, or GST-p53 was detected by Western blotting with anti-GST (Lower). (E) Full-length SIRT1, an N-terminal fragment (aa 1–244), a middle fragment (aa 245–498), and a C-terminal fragment (aa 499–747) were cloned into pGEX (Left), expressed in and purified from bacteria, and incubated with HEK293T cell lysates. Western blotting was performed to detect the interaction of Set7/9 with SIRT1 (Right).