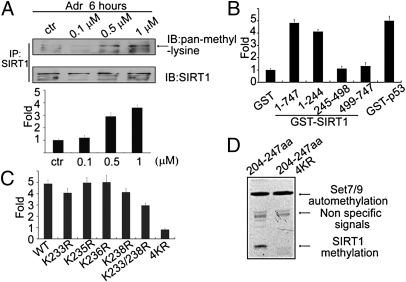
Fig. 3.
SIRT1 is methylated at multiple lysines in vitro and in vivo. (A) HCT 116 (p53+/+) cells were treated with increasing amounts of Adr (0.1, 0.5, 1 μM). Proteins were extracted for co-IP with anti-SIRT1 and probed with anti–pan-methyl-lysine (Upper). Methylation bands were scanned, and the relative band intensities were normalized to each SIRT1 band. The band intensity of the control was set as 1.0, and the numerical value of the intensity of each band was compared with the control (Lower). Data are means ± SD (n = 3). (B) GST-fusion fragments of SIRT1 (indicated in Fig. 2F) were purified from Escherichia coli, and an equal amounts were incubated with His-Set7/9 in HMT buffer at 30 °C for 3 h in the presence of 3H-SAM. The cpm value of each sample was measured by a scintillation counter. Data are means ± SD (n = 3). (C) Peptides including WT (PKRKKRKDIN), K233R (PRRKKRKDIN), K235R (PKRRKRKDIN), K236R (PKRKRRKDIN), K238R (PKRKKRRDIN), K233/238R (PRRKKRRDIN), and 4KR (PRRRRRRDIN) were incubated with His-Set7/9 in the presence of 3H-SAM and the signals were measured by using a scintillation counter. (D) A GST-fusion protein containing SIRT1 (aa 204–247) or mutated SIRT1 (aa 204–247; 4KR) was incubated with His-Set7/9 in the presence of 3H-SAM and exposed to film.