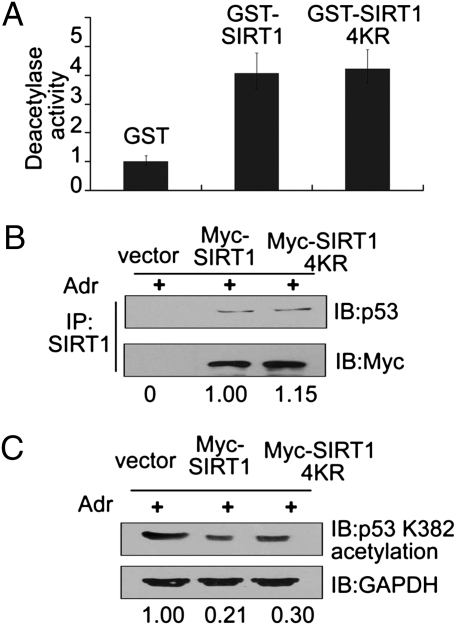
Fig. 4.
Methylation of SIRT1 is dispensable for its deacetylation activity. (A) GST-SIRT1-WT and GST-SIRT1-4KR mutant fusion proteins were generated and purified. The purified SIRT1 proteins were incubated first with Set7/9 for 2 h and then with acetylated p53 in the presence of NAD+. Deacetylase activity was then measured with an anti-acetylated p53 (K382). (B) A Myc WT-SIRT1 or a Myc SIRT1-4KR plasmid was transfected into HCT116 (p53+/+) cells and, after 24 h, cells were treated with 1 μM Adr for 6 h. Proteins were extracted for co-IP with anti-SIRT1 and probed with anti-p53 (Upper). The acetylated p53 bands were scanned and relative values are indicated below each band. The cell sample transfected with Myc-SIRT1 serves as the control and its value is set as 1 (Lower). (C) Under the same treatment conditions as in B, cells were harvested and subjected to Western blotting with anti-acetylated p53 (K382). GAPDH was the loading control. The acetylated p53 bands were scanned, and relative values are indicated below each band. The cell sample transfected with vector serves as the control, and its value is set as 1.