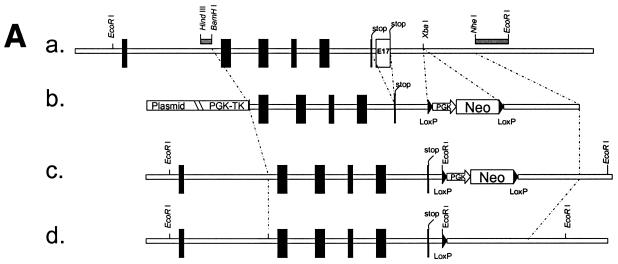
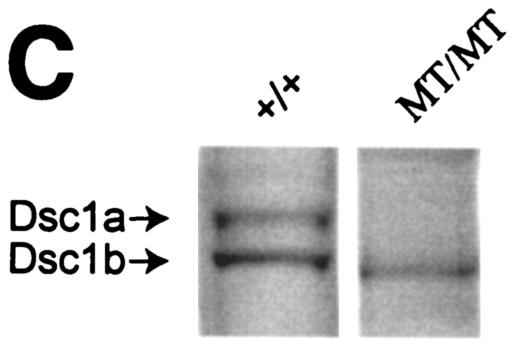
FIG. 1.
Strategy used to generate dsc1−/−ΔΕ17LoxP mutant mice. (A) Schematic representation of the targeting strategy used in this study. (a) The 3′ end of the mouse dsc1 gene locus is shown. Exons are represented by vertical bars. Exons 16 and 17 contain stop codons. Probes used to identify recombinant ES cell clones are shown as horizontal bars (HindIII/BamHI and NheI/EcoRI fragments). (b) Targeting construct TDSC1aΔE17. Intron 16 and exon 17 were deleted in this construct. A neomycin resistance minigene with flanking loxP sequences was inserted downstream of exon 17. The targeting vector also contained a thymidine kinase (TK) cassette. (c) Homologous recombination in ES cells generated the dsc1ΔE17Neo gene locus, in which intron 16 and exon 17 were deleted. (d) The neomycin resistance minigene was deleted through transient expression of CRE recombinase, generating the dsc1ΔE17LoxP allele. (B) RNase protection assays to detect Dsc1 RNA. Desmoglein 3 (left panel) and β-actin (right panel) were used as internal controls. Probes derived from dsc1 exon 17 (E17) and the 5′ end of the Dsc1 mRNA (5′), respectively, were used. Homozygous dsc1−/−ΔE17LoxP mutants express a Dsc1 RNA that does not contain exon 17 sequences. The genotype of the samples is indicated above the lanes (MT, dsc1−/−ΔE17LoxP mutant; +/+, wild type). Note that the expression levels in homozygous mutant mice are slightly lower than those in wild-type mice. (C) Western blot analysis using whole-skin extracts from wild-type (+/+) and homozygous mutant (MT/MT) mice with antibody gp899 (Dsc1). The positions of Dsc1a and Dsc1b in the wild-type sample are indicated. Note the absence of the Dsc1a band in the mutant sample.