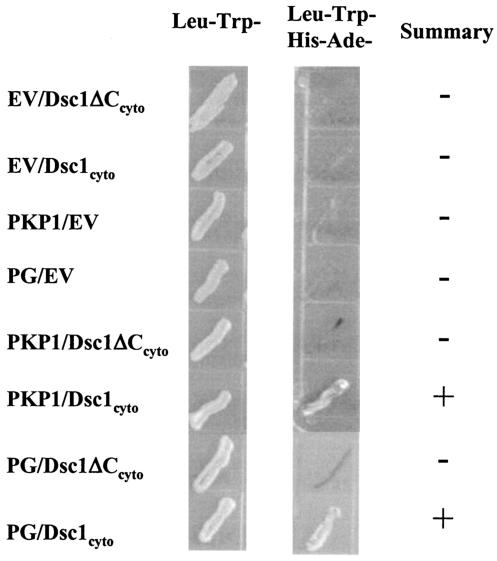
FIG. 3.
Molecular interactions of the cytoplasmic domains of Dsc1a (Dsc1cyto) and the truncated Dsc1 receptor (Dsc1ΔCcyto) synthesized in dsc1−/−ΔE17LoxP mutant mice. Yeast two-hybrid experiments were carried out to test for direct interactions by using growth in the absence of histidine and adenine (middle column) as a reporter for interactions between the cytoplasmic domains of the two Dsc1 molecules and several components of the desmosomal plaque. The cytoplasmic domain of Dsc1a, but not the COOH-terminally truncated Dsc1 mutant, interacts directly with the head domain of PKP1 and plakoglobin. Both Dsc polypeptides (Dsc1cyto and Dsc1ΔCcyto) were expressed as fusion proteins with the Gal4 activation domain and tested for interactions with either empty DNA binding domain vector (EV), the head domain of PKP1, or plakoglobin (PG) cloned into the Gal4 DNA binding domain vector.