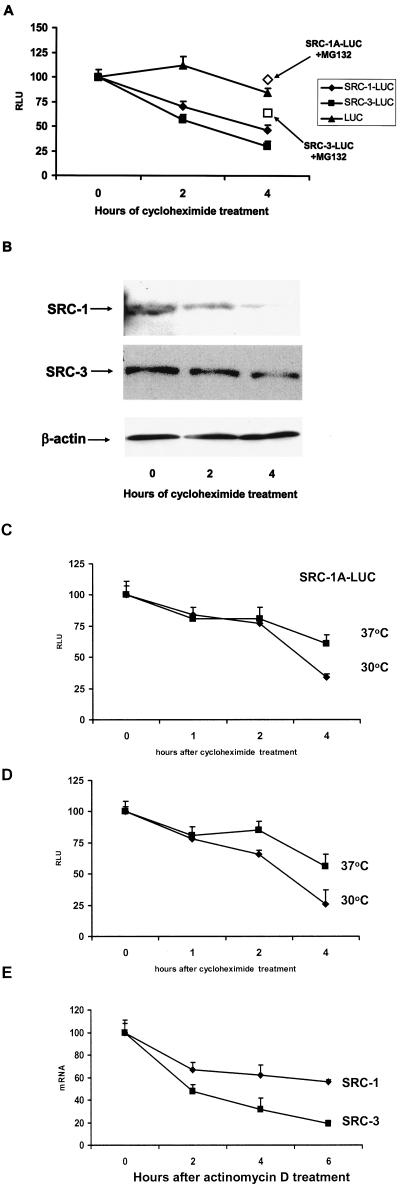
FIG.2.
Decay rate of SRC-1 and SRC-3 proteins. (A) HeLa cells were transiently transfected with expression vectors for SRC-1A-LUC, SRC-3-LUC, or luciferase along with pERE-E1b-CAT and pCR3.1 hERα. Twenty-four hours thereafter, cells were harvested just prior to treatment (0) or treated with cycloheximide (and MG132 [open boxes]; 4-h treatment time) at the zero hour time point and harvested 2 or 4 h thereafter for luciferase assays. (B) Decay of endogenous SRC-1A and SRC-3 in HeLa cells. HeLa cells were treated with cycloheximide, harvested at 0, 2, or 4 h thereafter, and visualized by Western analysis. As a loading control for the SRC-3 Western analysis, the blot was reprobed with β-actin (equal loading of SRC-1 was confirmed by the observation of nonspecific bands detected by the SRC-1 antibody [data not shown]). (C and D) SRC-1A-LUC and SRC-3-LUC are degraded in a proteasome-dependent manner in the temperature-sensitive UBA-defective ts85 cell line. Expression vectors for SRC-1A-LUC (C) and SRC-3-LUC (D) were transfected into ts85 cells. After 24 h of incubation at either the permissive (30°C) or restrictive (37°C) temperature, cells were then harvested at the time of cycloheximide treatment 1, 2, and 4 h thereafter and assayed for luciferase activity. (E) Decay rates for SRC-1 and SRC-3 in HeLa cells. HeLa cells were treated with the transcription inhibitor actinomycin D and harvested 0, 2, 4, and 6 h thereafter. SRC-1 and SRC-3 mRNA was quantitated by real-time PCR and normalized against 18S RNA.