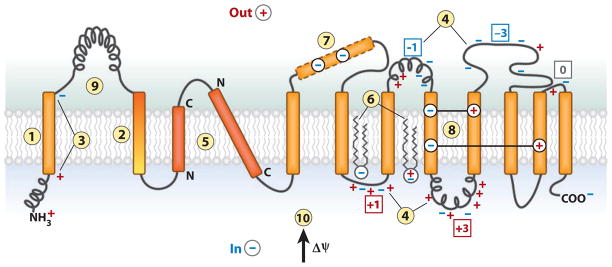
Figure 2.
The features of polytopic membrane proteins and biological membranes that determine folding and final transmembrane domain (TM) topology. TM integration is driven by (1) the hydrophobicity of TMs, and initial orientation is influenced by (2) the hydrophobic gradient (highest outward) along a TM, (3) the charge difference or (4) the positive-inside rule, (5) preferential orientation of highly hydrophobic TMs with the C terminus out if short and the N terminus out if long. After exit from the translocon, (6) final topology is influenced by lipid headgroup-protein charge interactions. During protein folding, interactions between TMs can stabilize (7) a domain containing charged residues as (8) a TM by compensating salt bridges. (9) A rapidly folding extramembrane domain can prevent reorientation of TMs during late folding events. (10) The positive outward membrane potential favors translocation of net negative domains and retention of net positive domains.