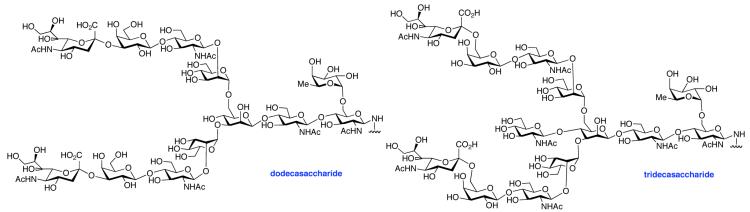
Table 2.
HOBT-mediated Aspartylation
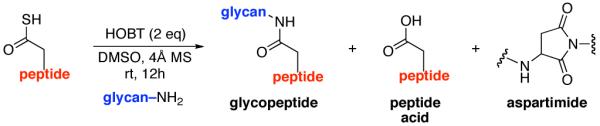
| entry | peptide + glycan ratio (P:G) |
product | yielda |
|---|---|---|---|
| 1 |
7 + 14 (1:2) No HOBT |
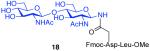
|
20% |
| 2 |
7 + 14 (1:1.5) |
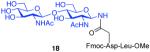
|
85%b |
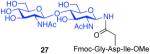
| 3 |
8 + 14 (1:2) |
|
75%c |
| 4 |
10 + 14 (1:2) |
|
52%d |
| 5 |
10 + 15 (1:1) |

|
50%e |
| 6 |
11 + 14 (1:2) |
|
55%f |
| 7 |
12 + 15 (1:1.5) |

|
52%g |
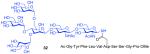
| 8 |
11 + 15 (1.5:1) |
|
75%h |
| 9 |
11 + 16 (1.8:1) |
|
49% |
| 10 |
11 + 17 (1.8:1) |
|
39% |
Isolated yield. The ratio of products was determined by LC-MS of the crude reaction mixture (product:acid:aspartimide)
89:10:1.
82:17:1.
59:37:4.
52:44:2.
60:35:5.
63:34:3.
62:34:4.