Figure 1.
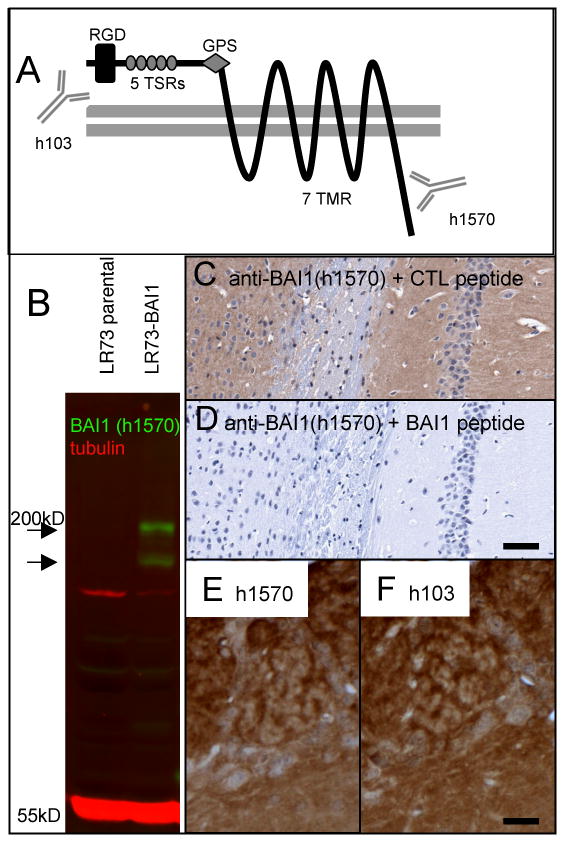
Demonstration of anti-BAI1 (h1570) antibody specificity. A) BAI1 is a 7-transmembrane (7 TMR) protein of the GPCR superfamily. The extracellular domain contains an RGD sequence, 5 thrombospondin type I repeats (TSRs) which are necessary for both the anti-angiogenic properties and apoptotic cell engulfment, and a G-protein coupled receptor (GPCR) proteolytic cleavage site (GPS). Antibody h103 is directed at an extracellular (N-terminal) sequence. The protein is cleaved at the GPS to release the anti-angiogenic fragment known as vasculostatin. The polyclonal h1570 antibody generated for the present study recognizes an amino acid sequence near the intracellular C-terminus. B) Western blotting of untransfected LR73 fibroblasts (parental) shows no detectable endogenous BAI1 signal (green) at the expected molecular weight of ∼180 kD. A stably transfected line (LR73-BAI1) shows strong h1570 blotting signal at the expected molecular weight as well as in a presumed proteolytic fragment. (C, D) Preincubation of diluted h1570 antibody with an irrelevant peptide (AMP-activated kinase, alpha-1, C-terminal peptide, Santa Cruz Biotechnology) had no effect on immunostaining of mouse hippocampus (C), whereas preincubation with the immunizing peptide completely abolished immunostaining (D). Immunohistochemistry on adjacent paraffin sections using the h1570 (C-terminal) and the h103 (N-terminal-directed) antibodies gave nearly identical staining patterns, with olfactory bulb shown as an example (E, F). Scale bars: D=100 μm; F=25 μm.
