Table 1.
Fluorination of alcohols.
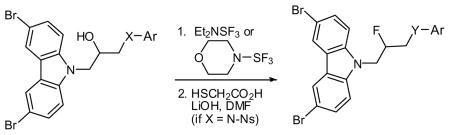 | |||||
|---|---|---|---|---|---|
| Entry | X | Ar | Y | Product | Yield (%)a |
| 1 | N(4-Ns)b | Ph | NH | 52 | 44 |
| 2 | N(4-Ns) | 3-(OCH3)-Ph | NH | 53 | 88 |
| 3 | N(4-Ns) | 4-(OCH3)-Ph | NH | 54 | 43 |
| 4 | N(4-Ns) | 4-(OCH2CO2Et)-Ph | NH | 55 | 61 |
| 5 | NCH3 | 3-(OCH3)-Ph | NCH3 | 56 | 71 |
| 6 | O | Ph | O | 57 | 97 |
| 7 | S | Ph | S(O)2c | 58 | 28 |
Isolated yield over one or two steps.
Ns = nitrobenzene sulfonyl.
After oxidation with m-CPBA
