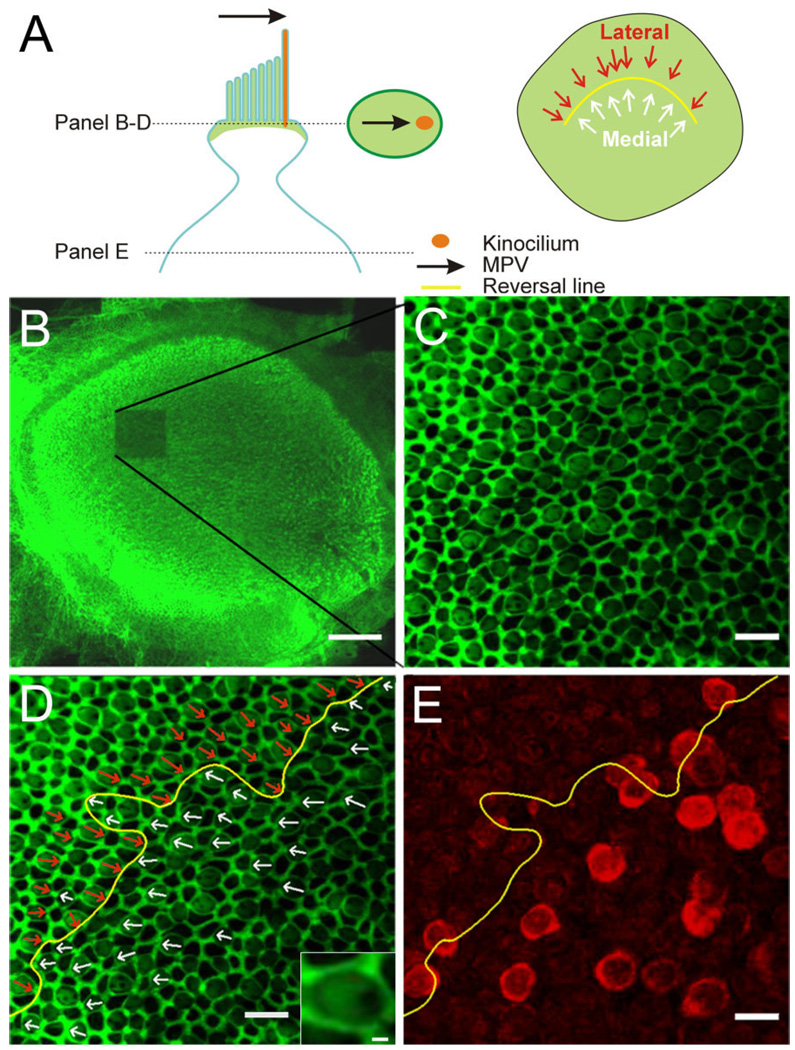
Figure 2.
Determination of the morphological polarization vector (MPV) and topographic localization of BK-positive hair cells. A: Schematic of an apical portion of a hair cell with the MPV (black arrow) pointing toward the kinocilium (orange). Stereocilia and the cuticular plate contain actin and lateral tight/adherens-junctions are associated with actin (green). The oval in the middle of illustrates a confocal section through the upper part of a hair cell. Actin staining creates a bright green surround (tight and adherens junctions), a lighter “fill” of the surround (base of stereocilia and cuticular plate), and a void (base of kinocilium). The MPV can thus be determined (black arrow). The schematic on the right shows the orientation of individual MPVs superimposed onto the utricular epithelium; the “reversal line” separates the two orientations (yellow line). B: Low-magnification confocal image of a phalloidin-stained utricle after one stack of high-magnification images was acquired (note bleached region). C: Single confocal section at higher magnification. Note the honeycomb pattern of the tight and adherens junctions between cells and the fainter phalloidin staining in between, revealing a clear void, indicating the location of the kinocilium. D: Same image as in C but with the MPVs for centrifugal (white arrows) and centripetal (red arrows) hair cells superimposed. The reversal line is indicated in yellow. Inset shows a magnified view of a hair cell to illustrate the “actin void” on the right side. E: A confocal section from the same stack but deeper into the tissue. The BK staining (red) is now apparent and the reversal line determined in D is superimposed. Note that because hair cells are not necessarily oriented orthogonal to the imaging plane, the location of the cell body does not give a clear indication of the MPV orientation for that hair cell. Scale bar = 100 µm in B; 10 µm in C–E; 1 µm in inset in D.