Abstract
Successful application of cross-linking combined with mass spectrometry for structural proteomics demands specifically designed cross-linking reagents to address challenges in the detection and assignment of cross-links. A combination of affinity enrichment, isotopic coding, and cleavage of the cross-linker is beneficial for detection and identification of the peptide cross-links. Here we describe a novel cross-linker, cyanurbiotindipropionylsuccinimide (CBDPS), that allows affinity enrichment of cross-linker-containing peptides with avidin. Affinity enrichment eliminates interfering non-cross-linked peptides and allows the researcher to focus on the analysis of the cross-linked peptides. CBDPS is also isotopically coded and CID-cleavable. The cleaved fragments still contain a portion of the isotopic label and can therefore be distinguished from unlabeled fragments by their distinct isotopic signatures in the MS/MS spectra. This cleavage information has been incorporated into a program for the automatic analysis of the MS/MS spectra of the cross-links. This allows rapid determination of cross-link type in addition to facilitating identification of the individual peptides constituting the interpeptide cross-links. Thus, affinity enrichment combined with isotopic coding and CID cleavage allows in-depth mass spectrometric analysis of the peptide cross-links. We have characterized the performance of CBDPS on the 120-kDa protein heterodimer of HIV reverse transcriptase.
Cross-linking combined with mass spectrometric analysis is an attractive technique for obtaining structural information on proteins and protein complexes (1). Cross-linked proteins can be enzymatically digested, and the cross-linked peptides (cross-links) obtained can be analyzed by mass spectrometry to identify both the cross-linked peptides and the site of cross-linking. Unfortunately, ion signals from the cross-links are usually overwhelmed by ion signals from non-cross-linked or “free” peptides and often difficult to detect and to assign. The most straightforward way of simplifying the mixture is by using cross-linking reagents with affinity tags such as biotin that allow selective enrichment of all of the cross-linker-containing peptides in the digest. Despite the added inconvenience of the synthesis, several biotinylated cross-linking reagents have been reported recently (2–6).
Even after the selective enrichment step, the detection of cross-links is still challenging. The most popular solution for facilitating specific detection of cross-links is isotopic coding of the cross-linking reagents (7). To enhance the signals from cross-links and to increase the likelihood of their identification, cross-links can be separated from the interfering free (non-cross-linked) peptides, thereby increasing their absolute and relative abundance and simplifying the subsequent mass spectrometric analysis. The final challenge results from the combinatorial nature of the possible interpeptide cross-links that complicates their assignment for complex protein systems even after affinity purification. This can be addressed by using cleavable cross-linkers, allowing sequencing of the individual peptides comprising the interpeptide cross-link. It would therefore be desirable to combine all of these useful features into a single cross-linking reagent that would simultaneously facilitate detection, enrichment, and identification of cross-links.
Cross-linking experiments normally produce a considerable number of cross-links (over 100 cross-links for a midsize protein), most of which are the less informative dead-end cross-links (attached through only one reactive group) and intrapeptide cross-links (two cross-linked residues within one peptide) instead of the more informative interpeptide cross-links that provide distance information. Rapid and automatic discrimination of cross-link type is therefore another desirable feature of a combined cross-linking/mass spectrometry analysis.
To discriminate dead-end from interpeptide cross-links using cross-linking reagents, several methods have been proposed, including conducting the cross-linking reaction in H218O water (2, 8), leading to incorporation of the oxygen atom from water during the hydrolysis of the unreacted active group of the cross-linker, which leads to a characteristic 2-Da shift for dead-end cross-links. This type of isotopic coding has been successfully combined with biotin tagging (2, 3), enabling the researcher to focus the analysis on the affinity-purified cross-links. Isotopic coding of the cross-linking reagents has also been combined with cleavage of the cross-linkers to allow the researcher to distinguish between the three cross-link types and to identify the individual peptides forming the cross-link (9, 10). Because the cleaved fragments are still isotopically coded, they can be easily detected in the spectra, and their relationships to the uncleaved parent cross-link can be determined based on mass differences. Cleavage of cross-linkers can be either done chemically (10), photoinduced (10), or done using CID (11–13). CID cleavage of cross-links has the advantage that the cleavage reaction occurs inside the mass spectrometer and can be performed individually by automatically mass-selecting each cross-link using an “include list.”
The combination of affinity purification with isotopic coding and CID cleavage enables automated comprehensive mass spectrometric analysis of a large number of peptide cross-links. Here we describe a novel cross-linker that combines all three of these features for the rapid automated analysis of the peptide cross-links: isotopic coding, CID cleavage, and biotin affinity tagging of the cross-linker. These three features ensure effective enrichment, confident detection, rapid automated cross-link type determination, and identification of the individual peptides composing each interpeptide cross-link. Here we describe this isotopically coded CID-cleavable biotinylated cross-linker, cyanurbiotindipropionylsuccinimide (CBDPS),1 as well as the specialized software (ICCLMSMS) that has been developed for the automated processing of the MS/MS spectra from these cross-links.
EXPERIMENTAL PROCEDURES
All materials were from Sigma-Aldrich unless noted otherwise.
Synthesis of CBDPS
0.1 mmol of cyanuric chloride was incubated with 0.1 mmol of hydrazidobiotin in 50% acetone or 1:1 acetone:D2O for 30 min on ice. To these reaction mixtures, solutions containing 0.2 mmol of mercaptopropionic acid prepared from either bromopropionic acid-H4 or bromopropionic acid-D4 (C/D/N Isotopes) and thiourea (10) were added. Reaction mixtures were neutralized by addition of 10 m NaOH or NaOD, respectively, and incubated at 75 °C for 30 min in open air. Residues were brought to 1 ml with water or D2O, and cyanurbiotindipropionate was precipitated by the addition of hydrochloric acid. The precipitates were washed twice with water or D2O and dried in vacuo. The resulting dipropionic acids were activated in dimethyl sulfoxide with N-hydroxysuccinimide in the presence of dicyclohexylcarbodiimide. The reaction mixtures were then filtered, and the combined filtrates were partitioned in 1:10 chloroform:water. The chloroform layer was collected and dried in vacuo. The overall yield was 30%. A 1:1 molar ratio mixture of CBDPS-H8/D8 is now available from www.creativemolecules.com.
Cross-linked Peptides
Model cross-linked peptides were prepared by incubating the synthetic peptide Ac-TRTESTDIKRASSREADYLINKER (Creative Molecules Inc.) with an equimolar amount of CBDPS-H8/D8 followed by enzymatic digestion with trypsin. The resulting peptide mixture was separated by reversed-phase HPLC. 1-ml fractions were collected and analyzed by MALDI-MS. Fractions containing dead-end and interpeptide cross-links were used for the affinity enrichment experiments.
Affinity Enrichment of CBDPS Cross-links
A 1-μl aliquot of the chromatographic fraction containing the interpeptide Lys-Lys cross-link TESTDIKR-CBDP-EADYLINKER was mixed with 10 μl of a 1 mg/ml tryptic digest of bovine serum albumin (BSA) in phosphate-buffered saline (PBS), pH 7.2. The mixture was affinity-purified with 10 μl of monomeric avidin-agarose bead slurry (Pierce). Beads were washed with PBS and then with water, and the affinity-bound material was eluted with 0.1% TFA and with 0.1% TFA, 50% acetonitrile. Aliquots from the loading, flow-through, wash, and elution fractions were desalted using C18 Zip-Tips (Millipore) and were analyzed by MALDI-MS.
Cross-linking Analysis of HIV Reverse Transcriptase
A 10-μl aliquot of a 1 mg/ml solution of HIV reverse transcriptase (HIV-RT) (Worthington Biochemical Corp.) in PBS was mixed with 1 μl of a 0.5 mm CBDPS-H8/D8 solution in water prepared from a 50 mm stock solution of the cross-linker. The final concentration of the cross-linker reagent was chosen based on the preliminary titration of the reaction mixture where the intensity of the cross-linked heterodimer band on SDS-PAGE was optimized without the appearance of any higher molecular weight bands from nonspecific higher oligomeric cross-linked products. The pH of the mixture was adjusted to 8.0–8.5 by the addition of 0.2 m Na2HPO4. The reaction mixture was incubated for 30 min at 25 °C and dialyzed against 50 mm ammonium bicarbonate. The cross-linked proteins were then digested with sequencing grade trypsin (Promega) overnight at 25 °C at a 10:1 substrate:enzyme ratio. The resulting peptide mixture was affinity-purified using monomeric avidin beads (Pierce) as described above for the test peptide. The elution fraction was concentrated by lyophilization and separated by nanoflow reversed-phase HPLC on a 1D Tempo nano-LC system (Eksigent) equipped with an LC Packings 0.3 × 5-mm C18 PepMap guard column (5-μm particle size, 100-Å pore size) and a 75-μm × 15-cm capillary column packed in house with Magic C18 AQ (Michrom Bioresources Inc.) particles (5 μm, 100 Å). This capillary LC system was operated at a flow rate of 300 nl/min using a 55-min gradient from 5 to 60% acetonitrile (0.1% TFA). The column effluent was spotted at 1-min intervals (300 nl/spot) onto a stainless steel MALDI target using a Dionex Probot spotter. The spots were dried; overlaid with 1 mg/ml α-cyano-4-hydroxycinnamic acid matrix solution in 0.1% TFA, 50% acetonitrile; and analyzed by MALDI-MS and -MS/MS using a 4800 MALDI-TOF/TOF system (Applied Biosystems). MS/MS spectra were acquired using “CID off,” 50 full-width half-maximum gate width, and a 1-kV MS/MS method. In cases where additional fragmentation was desirable for the unambiguous cross-link assignments, spectra were reacquired using “CID on” and a 2-kV MS/MS method.
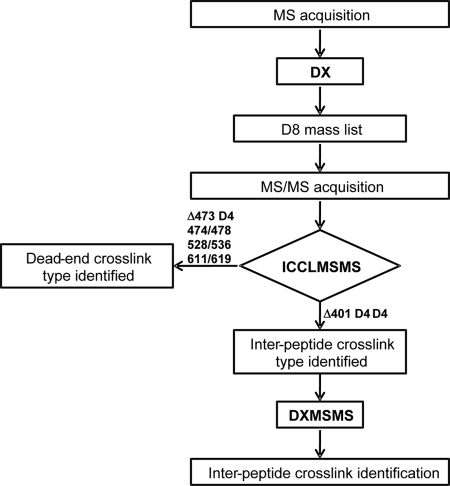
The mass spectra were analyzed using the ICC-CLASS software (14) and the ICCLMSMS program (http://www.creativemolecules.com/CM_Software.htm). The MS-Bridge program (http://prospector.ucsf.edu) with C19H23N7O4S3 as the elemental composition for the bridge can be used as an alternative to give possible assignments of a given cross-link mass.
The mass spectra from each chromatographic fraction were searched for D8 doublets using the DX program of ICC-CLASS. The D8 mass list obtained was used as an inclusion list for automatic MS/MS spectrum acquisition. The acquired MS/MS spectra were searched for isotopic signatures characteristic of cross-links using the ICCLMSMS program. Those cross-links, which were determined to be interpeptide cross-links by the ICCLMSMS software, were assigned using the DXMSMS component of the ICC-CLASS software package (Scheme 1).
Scheme 1.
Work flow of data analysis in CBDPS cross-linking experiment.
RESULTS AND DISCUSSION
Synthesis of CBDPS Cross-linker
For the synthesis of the cross-linker, we took advantage of the differential reactivity of the trifunctional cyanuric chloride. We were able to achieve the conjugation of hydrazidobiotin with cyanuric chloride in an equimolar reaction mixture without considerable formation of the di- and tribiotin products. Two residual chlorine atoms of the resulting dichlorocyanurhydrazidobiotin were substituted with mercaptopropionic acid, which was obtained by reaction of non-deuterated or deuterated bromopropionic acid with thiourea, followed by hydrolysis with sodium hydroxide. The obtained dipropionic acid was activated with N-hydroxysuccinimide in the presence of dicyclohexylcarbodiimide. This resulted in a reasonably compact symmetrical cross-linking reagent with a maximum span of 14 Å labeled with eight deuterium atoms (Fig. 1). The reactivity of the reagent is determined by the N-hydroxysuccinimide ester functional group, which primarily reacts with the primary amine groups on lysines and the N termini (15, 16).
Fig. 1.
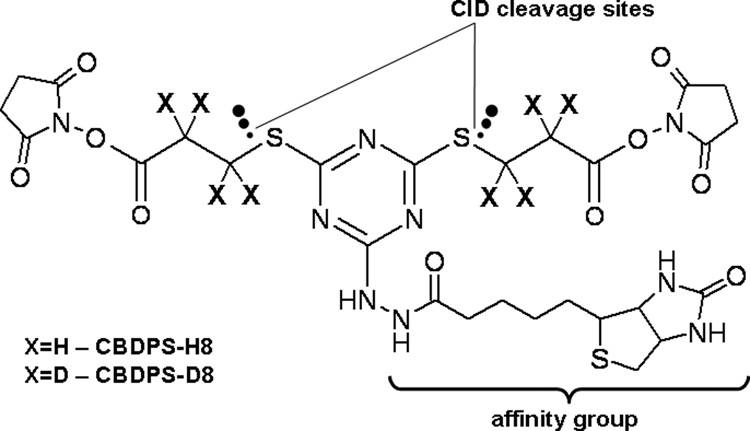
Structure of CBDPS. The bracket denotes the affinity biotin group. The dotted arrows indicate CID cleavage sites.
Peptide Cross-link Affinity Enrichment
The biotin-avidin interaction is traditionally used for specific enrichment of molecules of interest from complex biological mixtures. Placing a biotin group into the structure of the cross-linker reagent provides an affinity tag that can be used for specific pulldown (with avidin or streptavidin) of all the molecules containing a cross-linker moiety. Cross-linking of proteins with a biotinylated cross-linker followed by enzymatic digest leads to formation of a complex peptide mixture with all of the cross-linker-containing peptide species tagged with biotin. Adsorption of biotinylated peptides onto immobilized avidin followed by washing away unbound peptides and the subsequent elution of the biotinylated peptides results in specific purification of the peptide cross-links. The benefits of this procedure for the following mass spectrometric analysis are 2-fold: first, the elimination of ion suppression effects by removing the majority of the more abundant free peptides, and second, the specific enrichment and concentration of the peptide cross-links. Proof-of-principle affinity purification of the CBDPS-cross-linked test peptide showed, as expected, that this purification step eliminated most of the interfering free peptides. This led to a strong signal for the cross-link in the elution fraction, whereas it was undetectable in the load peptide mixture (data not shown). The simplified MS spectrum of the affinity-purified fraction allows unobstructed subsequent MS/MS analysis of the peptide cross-links.
The same tendency was observed in the case of the HIV-RT cross-link enrichment: no doublets were detected in the load fraction of the cross-linked proteins digest, whereas over 30 doublets were detected in the elution fraction from the avidin column. Many of the doublets overlapped in the single spot mass spectrum, which necessitated additional HPLC fractionation of the digest mixture.
CID Cleavage of Cross-linker
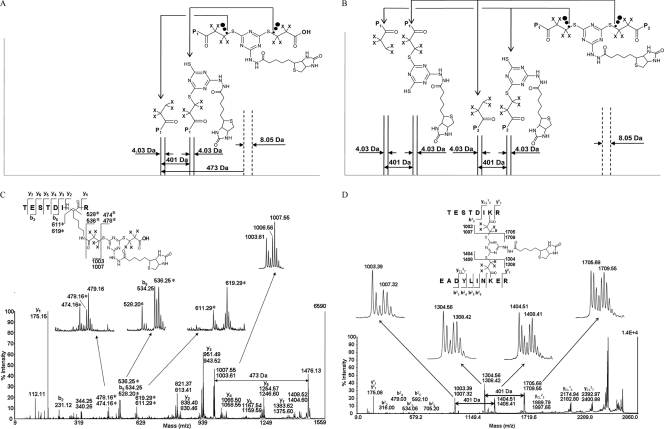
MS/MS analysis of the model interpeptide CBDPS cross-link revealed that cleavage of the cross-linker bridge was the predominant cleavage under MALDI and ESI MS/MS CID conditions (Fig. 2 and supplemental Figs. 1–3). The cleavage occurs distal to the cyanuric group C–S bonds. Due to the cleavage of the C–S bond, dead-end cross-links can be distinguished by a prominent doublet of signals 4 Da apart in the MS/MS spectra, at a mass 473 Da lower than the precursor mass, which corresponds to a peptide that contains the residual thiopropionic moiety of the cross-linker (Fig. 2A). The characteristic 474/478-Da doublet corresponds to the cleaved off portion of the hydrolyzed cross-linker. This doublet often contains an abundant m/z 479 ion that results from the 13C isotope of the 478 ion plus proton/deuterium exchange during bond cleavage as described below. Additional high abundance signals found in the MS/MS spectra of dead-end cross-links were an 8-Da doublet at m/z 611/619, corresponding to the immonium ion modified with the hydrolyzed cross-linker lysine residue, and an 8-Da doublet at m/z 528/536, corresponding to the cross-linker that was cleaved at the isopeptide bond of the lysine side chain (Fig. 2C) (the m/z 535 and m/z 618 signals originate from the D7 form of the cross-linker, which overlaps with the isotope pattern from the m/z 534 b5 fragment ion).
Fig. 2.
CID cleavage of CBDPS cross-links. A, CID cleavage of CBDPS dead-end cross-links. B, cleavage of interpeptide CBDPS cross-links. P1 and P2 are the cross-linked peptides. C, CID cleavage of dead-end CBDPS cross-links from the test peptide. The characteristic ions for the isotopically coded fragments from the dead-end cross-links are marked by asterisks. D, CID cleavage of the CBDPS interpeptide cross-link from the test peptide.
Close examination of these doublets revealed that although the fragment ions from the light version of the cross-linker displayed the expected isotope pattern, there were anomalies in the isotope patterns of the fragment ions containing the heavy form of the cross-linker. Comparison of “pairs” of ions from the two portions of the cross-link (for example, m/z 1006/1007 and m/z 1709/1710 in Fig. 2D) showed that proton/deuterium exchange often occurs between the two portions of the cross-linker but can only be observed in the deuterated form of the cross-linker where it leads to an unusual but characteristic isotopic distribution which contains an (M −1 Da) peak for the fragment that does not include the nitrogen-containing ring and a higher than expected (M + 1 Da) peak in the corresponding cleaved portion of the cross-link that includes the nitrogen-containing ring. Although the details of the CID cleavage of this cross-linker will be the basis of a future study, this phenomenon provides an additional and very specific “fingerprint” for identifying and assigning cross-link pairs.
Cleavage of the CBDPS cross-link creates a unique isotopic pattern of peaks from which the individual peptides constituting the interpeptide cross-link can easily be identified. Thus, this cross-linker fulfills the most important function of isotopically coded cleavable cross-linkers, allowing the identification of the linear peptides comprising the interpeptide cross-links. This greatly facilitates the cross-link assignments, which are the main bottleneck in cross-linking studies because of the combinatorial nature of interpeptide cross-links as mentioned above.
We have previously reported other chemically and photocleavable isotopically coded cross-linking reagents (9, 10). The advantage of CID-cleavable cross-linkers compared with other modes of cleavage is that each intact cross-link can be mass-selected and fragmented separately from the others in the mixture, thus allowing individual cleavage and mass spectrometric analysis of each cross-link, giving the masses of the cleaved peptides that formed the cross-link along with some sequence-specific fragments from each individual peptide. In addition, the CID-cleaved peptides can be further analyzed by MS/MS/MS, depending on the instrument. For example, use of an ion trap could provide additional sequence information on each individual peptide that formed the cross-link if needed.
ICCLMSMS Spectrum Analysis Program
The mass relationships between the fragment ion masses were used to create a program for the analysis of the MS/MS spectra of isotopically coded CID-cleavable cross-links, called ICCLMSMS. Using the mass values for CBDPS, an entire set of MS/MS spectra can be automatically searched for the presence of 1) m/z 611/619 immonium ions and m/z 528/536 and m/z 474/478 fragment ions from dead-end cross-links, 2) a pair of D4 doublets with one 473 Da and the other 72 Da lower in mass than the precursor ion mass that are characteristic for the dead-end cross-links, 3) pairs of D4 doublets 401 Da apart that correspond to the individual peptides resulting from CID cleavage of the cross-links, and 4) D4 doublet pairs 401 Da apart that add up to the precursor ion mass and that represent the individual cleaved peptides constituting the interpeptide cross-link. Criteria 1 and 2 are used to distinguish dead-end cross-links from interpeptide cross-links, and criteria 3 and 4 are used for identifying the interpeptide cross-links. Searching for this combination of criteria provides a fast and efficient means of identifying these cross-link types in an automated manner. The program can be used for any isotopically coded CID-cleavable cross-linker and is freely available on the Internet.
Cross-linking Analysis of HIV-RT Heterodimer
To assess the performance of CBDPS on the protein level, HIV-RT (a partial homodimer consisting of two subunits, 55 and 61 kDa) was cross-linked using this reagent. The cross-linking reaction mixture was quenched with ammonium bicarbonate, dialyzed, and digested overnight with trypsin. The dialysis step was found to be important for the removal of any excess biotinylated reagent and avoids any competition with cross-links during the subsequent affinity enrichment step. Another important consideration is the amount of avidin to be used for purification relative to the amount of the protein being cross-linked. To optimize capture of all of the biotinylated cross-links, the theoretical binding capacities of avidin or streptavidin and the number of lysine residues in the protein can be calculated, and equimolar amounts of avidin and lysine residues can be used as a starting point for the affinity purification step.
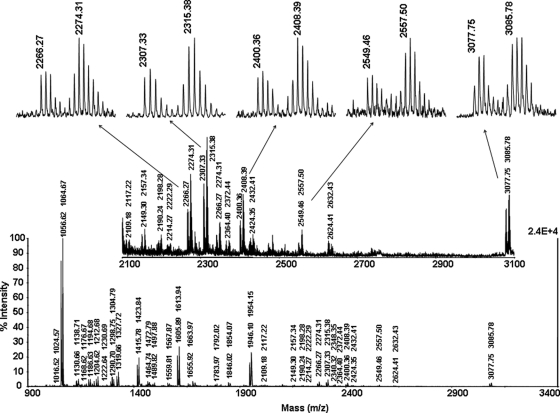
The resulting peptide mixture was then affinity-purified with monomeric avidin beads and analyzed by MALDI-MS. As in the case of the model peptide experiment, the procedure for affinity purification of the CBDPS HIV-RT cross-links led to enrichment of the cross-links in the sample and elimination of the unmodified peptides, thereby resulting in a spectrum essentially containing only signals from the cross-links, many of which were undetectable in the load fraction prior to the purification (two cross-links were detected without affinity enrichment, whereas 24 doublets were detected after affinity enrichment). Separation of the affinity-purified cross-links by HPLC further improved the detection of the cross-links probably by reducing supplementary suppression effects. The mass spectra of the HPLC fractions contain virtually only signals from cross-links (Fig. 3).
Fig. 3.
Mass spectrum of HPLC fraction of affinity purified CBDPS HIV-RT cross-links. Mass values of isotopically coded CBDPS cross-links peak doublets are represented as pairs of aligned numbers.
Mass spectra of the chromatographic fractions were then searched for doublets using the DX program within the ICC-CLASS software suite. The resulting D8 doublet mass list was used as the inclusion list for automatic acquisition of MS/MS spectra. A total of 78 D8 doublets were detected and analyzed by MS/MS. The MS/MS spectra were searched with the ICCLMSMS program to flag immonium ions and hydrolyzed cross-linker fragments signals for dead-end cross-links and to identify the CID-cleaved portions of interpeptide cross-links. The dead-end cross-links were omitted from further analysis, and the interpeptide cross-link candidates were assigned on the basis of the cross-link mass, the masses of the individual peptides derived from the CID cleavage, and some sequence-specific ions using the DXMSMS program within ICC-CLASS, which was updated with parameters for CBDPS. Of the 78 D8 doublets detected, 48 unique cross-links masses were identified: 38 as dead-end, five as tryptic interpeptide, three as non-tryptic interpeptide, and two as interpeptide cross-links that were not unambiguously assigned (Table I).
Table I. HIV-RT CBDPS tryptic interpeptide cross-links.
D8 obs and D8 calc, observed and calculated D8 peak doublets, respectively; Δ, mass error for D8 peak doublet assignments; RS and RE, starting and ending amino acid residues (sequence numbers) of the cross-linked peptides, respectively; D4 1 and D4 2, D4 peak doublets of cleaved products, respectively.
| D8 obs doublet | D8 calc doublet | Δ | HPLC fraction | RS sequence number | RE sequence number | Amino acid sequence | D4 1 doublet | D4 2 doublet |
|---|---|---|---|---|---|---|---|---|
| Da | Da | ppm | Da | Da | ||||
| 1575.65 | 1575.70 | 31.7 | 25 | 65 | 66 | K↓KK↓Da | 329.20 | 730.27 |
| 67 | 72 | K↓DSTKWR↓D | 846.39 | 1247.41 | ||||
| 1787.88 | 1787.85 | −16.8 | 26 | 79 | 83 | R↓ELNKR↓T | 713.36 | 1114.41 |
| 386 | 390 | K↓TPKFK↓L | 674.37 | 1075.38 | ||||
| 1961.92 | 1961.88 | −20.4 | 26 | 67 | 72 | K↓DSTKWR↓D | 846.35 | 1247.45 |
| 44 | 49 | R↓EGKISK↓T | 715.31 | 1116.40 | ||||
| 2109.91 | 2109.98 | 33.2 | 25 | 12 | 20 | K↓LKPGMDGPK↓V | 996.46 | 1397.57 |
| 79 | 83 | R↓ELNKR↓T | 713.37 | 1114.45 | ||||
| 3077.75 | 3077.44 | −100.7 | 28 | 351 | 356 | K↓TGKYAR↓K | 749.30 | 1150.39 |
| 308 | 323 | R↓EILKEPVHDVYYDPSK↓D | 1927.96 | 2329.07 |
a This could also be aa 102/103 or aa 103/104 on the basis of mass but not when distances between these residues in the crystal structure are considered.
The identified cross-links were mapped to the crystal structure of the HIV-RT dimer (Protein Data Bank code 1DLO:(17)). All the distances between the identified cross-linked sites were in good agreement with the length of the cross-linker and with our previous results (18), thus providing validation of this new cross-linking reagent. Although many of the cross-links were already known from our previous work, two new cross-links were identified using this new reagent. Interestingly, although many of the other cross-links could come from either chain, the length of one newly discovered aa 82–388 cross-link (11.4 Å) is compatible only with the B chain and not the A chain where the distance between these two residues would be 53.8 Å. Similarly, the previously discovered bis(sulfosuccinimidyl) suberate (BS3) cross-link between aa 82 and aa 287 can only be spanned in the A chain, whereas the BS3 cross-link between aa 70 and aa 220 can only be spanned in the B chain. This discrimination, of course, is possible only because the conformations of the two forms of the subunits were already known. CBDPS showed three possible new cross-links connecting aa 70 to a “KK” sequence that could be aa 65/66, aa 102/103, and/or aa 103/104 in HIV-RT (see Table I). However, only one of these, aa 65/66, is within the possible distance spanned by CBDPS, and this cross-link can occur in either chain.
Overall, the combination of the specific features of CBDPS makes this reagent very attractive for cross-linking applications. Affinity-purified cross-links can be distinguished on the basis of their isotopic signatures in the spectra and cleaved by CID to produce individual peptides that are readily distinguishable from background fragments on the basis of their new isotopic signatures in the MS/MS spectra. We successfully validated this approach using LC-MALDI analysis of the digested cross-linked HIV-RT heterodimer. The method is potentially applicable in LC-ESI mode, although as in other labeling studies with deuterium attention needs to be paid to possible differences in retention time of the light and heavy isotopic forms of the cross-links. This problem may be eliminated in a future version of the cross-linker with 13C isotopic labels instead of deuterium.
Placing a CID-labile bond in the cross-linking bridge allows one to readily generate the masses of the individual peptides composing the interpeptide cross-links but, in turn, could possibly reduce fragmentation of the peptide backbone. The relative value of having individual peptide masses instead of backbone peptide fragments for cross-link identification is an interesting bioinformatics question. However, we have found that knowing the masses of the individual peptides, especially with high mass accuracy, addresses the main combinatorial problem with interpeptide cross-links and greatly reduces the number of possible assignments for a given cross-link. These assignments can be further strengthened by having peptide backbone fragment information, which can be obtained by higher energy CID fragmentation of the cross-links or by possible by MS/MS/MS analysis of the cleaved peptides.
Conclusion
Here we have described a novel cross-linker, CBDPS, for structural proteomics. CBDPS combines three of what we believe are critical features necessary for successful mass spectrometric analysis of the peptide cross-links: isotopic coding, cleavability, and affinity enrichment. Following affinity enrichment of cross-linked peptides using immobilized avidin, each cross-link can be individually cleaved by CID. Cleavage of the cross-linker leads to formation of easily identifiable isotopically coded forms of the peptides that formed the cross-link. This allows automated analysis of the MS/MS spectra for which the isotopic coding of the sequence ions greatly facilitates the assignment of the peptides.
The limitations of this approach are similar to those of any protein identification experiment. Limited amounts of a complex can lead to low abundance signals from the cross-links. For a multicomponent complex, there is also the possibility that overlapping components may mask true doublets or may produce “false-positive” doublet signals. However, the features of this cross-linker were designed to assist in the detection of these signals: first, by allowing affinity purification of the cross-links, and second, by allowing CID-based determination of the masses of the individual peptides making up the cross-link. In addition, multidimensional separation of the affinity-purified cross-link mixture is a useful option for highly complex mixtures. For several of our current projects (19), we are using a combination of gel separation, strong cation exchange, affinity purification, and reversed-phase separations that greatly assists in the detection of low abundance affinity-enriched cross-links.
Another limitation, which this cross-linker shares with other amine-reactive reagents, is that its use will lead to missed tryptic cleavages. This needs to be taken into account in the database searching. In addition, if there are multiple adjacent cleavage or reaction sites in the protein (as was the case for HIV-RT), it may not be possible to unambiguously localize the resulting cross-linking site.
Mass accuracy is always a key issue in cross-linking experiments. Because, for a given resolution, mass error/mass uncertainty is a function of mass, there is more uncertainty in the measurement of higher molecular weight peptides and cross-links than in measurements of lower molecular weight peptides. Mass measurements of intact cross-linked peptides may therefore carry more uncertainty in mass than do mass measurements on their cleaved components. This could be an advantage of using a cleavable cross-linker. However, depending on the instrument, this advantage may be offset by lower resolution in the CID mode.
In conclusion, we have found that this combination of affinity enrichment, isotopic coding, and CID cleavage allows confident identification of otherwise undetectable cross-links in digests of protein complexes. The agreement of the experimental results with the known protein structure of the HIV-RT heterodimer confirms the value of cross-linking combined with mass spectrometry for studying protein structures. The properties of the CBDPS cross-linking reagent, combined with automated mass spectrometric analysis and data processing, make this cross-linker a particularly useful and effective tool for protein structural studies.
Acknowledgments
We are thankful to Leanne Ohlund for assistance with nano-LC and Dr. Carol E. Parker for critical reading of the manuscript.
Footnotes
* This work was supported by a Genome Canada, Genome British Columbia technology development grant.
 This article contains supplemental Figs. 1–3.
This article contains supplemental Figs. 1–3.
1 The abbreviations used are:
- CBDPS
- cyanurbiotindipropionylsuccinimide
- aa
- amino acid(s)
- BS3
- bis(sulfosuccinimidyl) suberate.
REFERENCES
- 1. Sinz A. (2006) Chemical cross-linking and mass spectrometry to map three-dimensional protein structures and protein-protein interactions. Mass Spectrom. Rev. 25, 663–682 [DOI] [PubMed] [Google Scholar]
- 2. Chu F., Mahrus S., Craik C. S., Burlingame A. L. (2006) Isotope-coded and affinity-tagged cross-linking (ICATXL): an efficient strategy to probe protein interaction surfaces. J. Am. Chem. Soc. 128, 10362–10363 [DOI] [PubMed] [Google Scholar]
- 3. Hurst G. B., Lankford T. K., Kennel S. J. (2004) Mass spectrometric detection of affinity purified crosslinked peptides. J. Am. Soc. Mass Spectrom. 15, 832–839 [DOI] [PubMed] [Google Scholar]
- 4. Fujii N., Jacobsen R. B., Wood N. L., Schoeniger J. S., Guy R. K. (2004) A novel protein crosslinking reagent for the determination of moderate resolution protein structures by mass spectrometry (MS3-D). Bioorg. Med. Chem. Lett. 14, 427–429 [DOI] [PubMed] [Google Scholar]
- 5. Petrotchenko E. V., Thomas J. M., Borchers C. H. (2008) A collection of novel isotopically-coded crosslinkers for structural proteomics, presented at the 56th Annual Conference on Mass Spectrometry and Allied Topics, June 1–5, 2008, Denver CO, American Society for Mass Spectrometry, Santa Fe, NM [Google Scholar]
- 6. Kang S., Mou L., Lanman J., Velu S., Brouillette W. J., Prevelige P. E., Jr. (2009) Synthesis of biotin-tagged chemical cross-linkers and their applications for mass spectrometry. Rapid Commun. Mass Spectrom. 23, 1719–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Müller D. R., Schindler P., Towbin H., Wirth U., Voshol H., Hoving S., Steinmetz M. O. (2001) Isotope-tagged cross-linking reagents. A new tool in mass spectrometric protein interaction analysis. Anal. Chem. 73, 1927–1934 [DOI] [PubMed] [Google Scholar]
- 8. Seebacher J., Mallick P., Zhang N., Eddes J. S., Aebersold R., Gelb M. H. (2006) Protein cross-linking analysis using mass spectrometry, isotope-coded cross-linkers, and integrated computational data processing. J. Proteome Res. 5, 2270–2282 [DOI] [PubMed] [Google Scholar]
- 9. Petrotchenko E. V., Olkhovik V. K., Borchers C. H. (2005) Isotopically coded cleavable cross-linker for studying protein-protein interaction and protein complexes. Mol. Cell. Proteomics 4, 1167–1179 [DOI] [PubMed] [Google Scholar]
- 10. Petrotchenko E. V., Xiao K., Cable J., Chen Y., Dokholyan N. V., Borchers C. H. (2009) BiPS, a photocleavable, isotopically coded, fluorescent cross-linker for structural proteomics. Mol. Cell. Proteomics 8, 273–286 [DOI] [PubMed] [Google Scholar]
- 11. Soderblom E. J., Bobay B. G., Cavanagh J., Goshe M. B. (2007) Tandem mass spectrometry acquisition approaches to enhance identification of protein-protein interactions using low-energy collision-induced dissociative chemical crosslinking reagents. Rapid Commun. Mass Spectrom. 21, 3395–3408 [DOI] [PubMed] [Google Scholar]
- 12. Soderblom E. J., Goshe M. B.(2006) Collision-induced dissociative chemical cross-linking reagents and methodology: applications to protein structural characterization using tandem mass spectrometry analysis. Anal. Chem. 78, 8059–8068 [DOI] [PubMed] [Google Scholar]
- 13. Lu Y., Tanasova M., Borhan B., Reid G. E. (2008) Ionic reagent for controlling the gas-phase fragmentation reactions of cross-linked peptides. Anal. Chem. 80, 9279–9287 [DOI] [PubMed] [Google Scholar]
- 14. Petrotchenko E. V., Borchers C. H. (2010) ICC-CLASS: isotopically-coded cleavable crosslinking analysis suite. BMC Bioinformatics 11, 64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mädler S., Bich C., Touboul D., Zenobi R. (2009) Chemical cross-linking with NHS esters: a systematic study on amino acid reactivities. J. Mass Spectrom. 44, 694–706 [DOI] [PubMed] [Google Scholar]
- 16. Kalkhof S., Sinz A. (2008) Chances and pitfalls of chemical cross-linking with amine-reactive N-hydroxysuccinimide esters. Anal. Bioanal. Chem. 392, 305–312 [DOI] [PubMed] [Google Scholar]
- 17. Hsiou Y., Ding J., Das K., Clark A. D., Jr., Hughes S. H., Arnold E. (1996) Structure of unliganded HIV-1 reverse transcriptase at 2.7 A resoultion: implications of conformational changes for polymerization and inhibition mechanisms. Strucutre 4, 853–860 [DOI] [PubMed] [Google Scholar]
- 18. Petrotchenko E. V., Serpa J. J., Borchers C. H. (2010) Use of a combination of isotopically coded cross-linkers and isotopically coded N-terminal modification reagents for selective identification of inter-peptide crosslinks. Anal. Chem. 82, 817–823 [DOI] [PubMed] [Google Scholar]
- 19. Cabecinha A., Petrotchenko E., Borchers C. (2009) Out-gel Digest Procedure for Protein Cross-linking Applications, presented at the 57th Annual Conference on Mass Spectrometry and Allied Topics, Philadelphia, PA, May 31–June 4, 2009, American Society for Mass Spectrometry, Santa Fe, NM [Google Scholar]