Fig. 8.
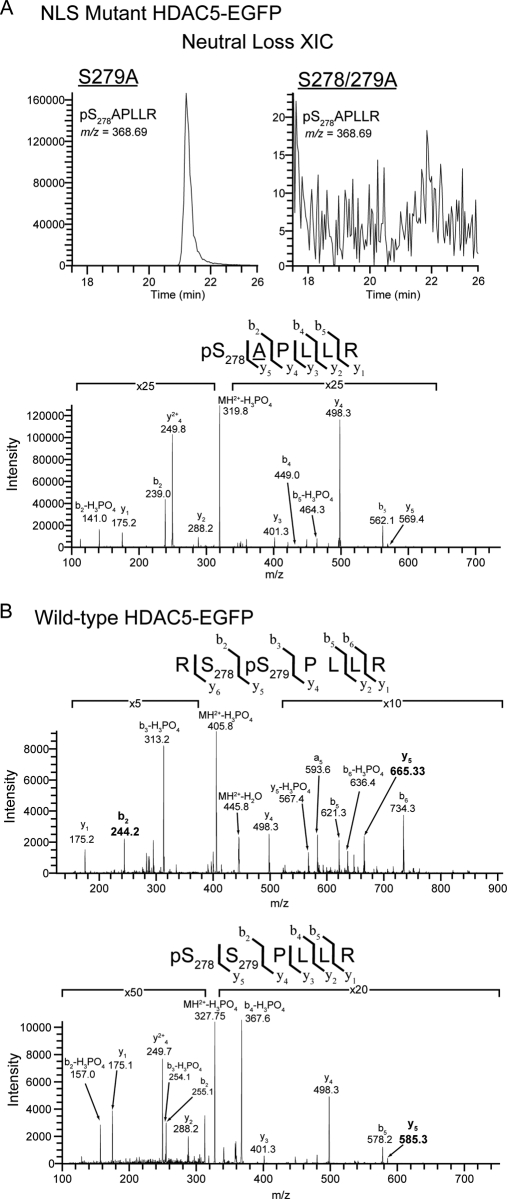
Targeted CID-MS2 analysis of NLS region demonstrated the independence of Ser278 phosphorylation from Ser279 phosphorylation. A, Neutral loss extracted ion chromatogram (NL-XIC) (top) and CID MS/MS spectrum (bottom) of the tryptic phosphopeptide pS278APLLR from the NLS region demonstrated the presence of Ser278 phosphorylation in S279A HDAC5-EGFP. This peptide ion (m/z = 368.69) was absent in the equivalent NL-XIC from S278/279A HDAC5-EGFP. B, Phosphopeptide CID spectra corresponding to doubly charged tryptic peptides from the NLS region of wild-type HDAC5 localized a single phosphorylation to Ser279 (top) and confirmed the previous identification of Ser278 phosphorylation by ETD (bottom). y and b ion fragments are indicated above each spectra, with site determining ions (b2 and y5) highlighted in bold.