Fig. 3.
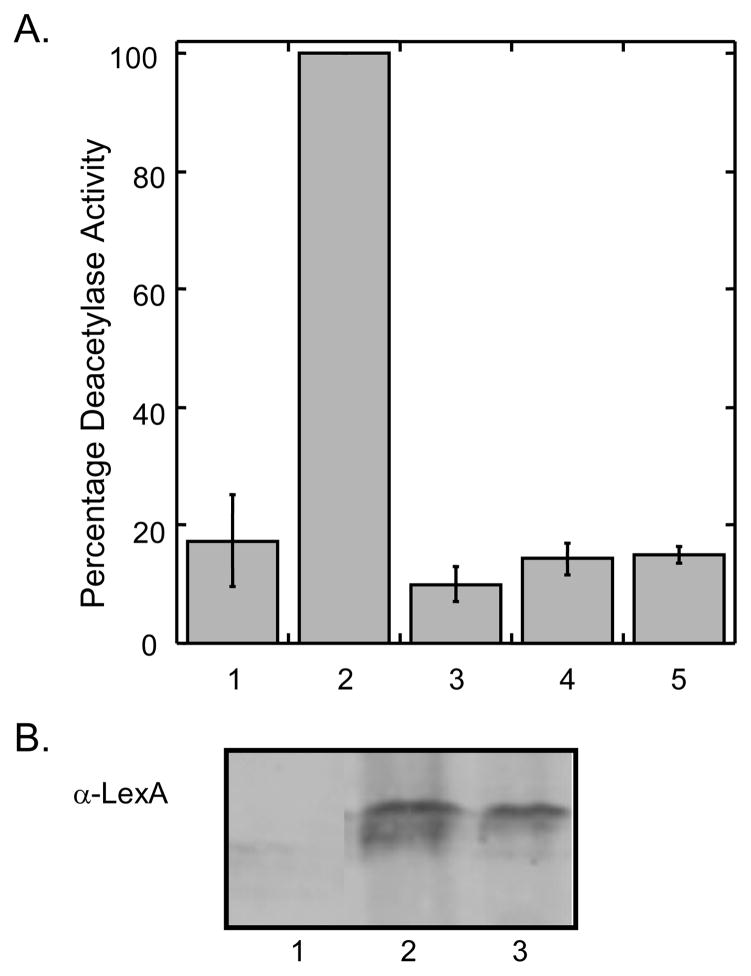
A. Deacetylase activity of Rpd3-LexA-FLAG fusions. Rpd3 (column 2 and 3) or Rpd3H150/151A (column 4 and 5) were expressed in FT5 Δrpd3::HIS3 yeast strain as LexA-FLAG fusion proteins and immunoprecipitated using their C-terminal FLAG tag and anti-FLAG-agarose resin (Sigma). A no protein control was included in column 1. The immunoprecipitated proteins were used in fluorescence HDAC assays either in the absence (columns 2 and 4) or presence (columns 3 and 5) of 100 μM trichostatin. The mean percent activities of three independent trials was compared with that of the wild-type protein (100%-column 2) with standard error shown (Table S1). B. The immunoprecipitated proteins were separated by SDS-PAGE and probed with a LexA antibody (Santa Cruz) to ensure protein expression. Lane 1: no protein control; lane 2: Rpd3-LexA-FLAG; lane 3: Rpd3H150/151A-LexA-FLAG.