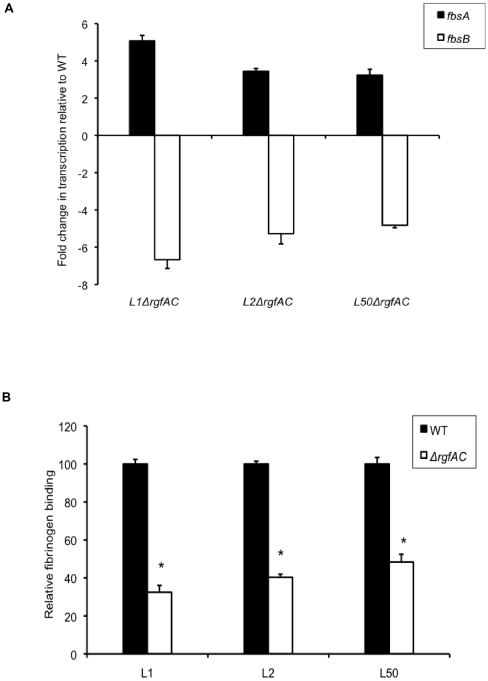
Figure 2. Properties of ΔrgfAC mutant strains.
(A) Fold change in transcription levels of fbsA (filled boxes) and fbsB (open boxes) genes in the isogenic ΔrgfAC mutants as compared to the wild type L1, L2, and L50 strains (WT). The amount of transcripts of each gene was normalized to the amount of gyrA transcripts and expressed relative to the level of transcription in corresponding WT strain. Each experiment was performed at least three times. Boxes are means and bars are standard deviation of the means. (B) Binding ability to immobilized human fibrinogen of the isogenic ΔrgfAC mutants (open boxes) and the WT strains (filled boxes). Flat bottomed 96-well polystyrene plates were coated with 21 nM human fibrinogen and 5×106 to 5×108 CFU per ml were added for 90 min at 37°C. Binding ability was calculated from the ratio between the number of bound bacteria and the number of bacteria present in the inoculum. The level of fibrinogen binding of WT strains is arbitrarily reported as 100 and the fibrinogen-binding levels of the isogenic mutants are relative values. Each experiment was performed at least three times. Boxes are means and bars are standard deviation of the means. * indicates that the binding values of the mutant strains were significantly lower than the values of the corresponding WT strains, at a P value of <0.001.