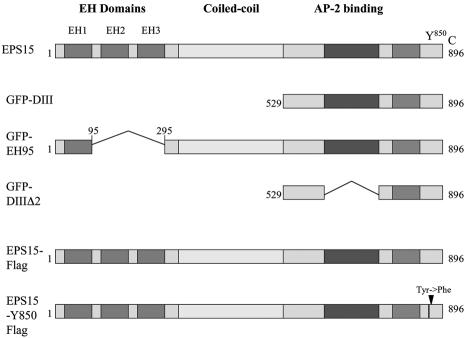
FIG. 4.
Schematic representation of full-length eps15 and the dominant-negative mutant constructs used in these experiments. eps15 has an amino terminus containing three protein-protein interaction EH domains, a central coiled-coil domain thought to be involved in homodimerization, and a carboxyl terminus involved in association with the clathrin adaptor protein AP-2 during clathrin pit formation. The DIII (D3) and EH95 constructs are dominant-negative inhibitors of eps15 function and inhibit clathrin-dependent endocytosis, whereas the DIIIΔ2 (D3D2) construct serves as a negative control and has no effect on clathrin-dependent endocytosis. The FLAG constructs are either a control full-length eps15 construct or full-length eps15 with a point mutation (Tyr→Phe) in the major tyrosine phosphorylation site, Y850, which has been shown to block EGF internalization but not transferrin internalization.