Figure 3.
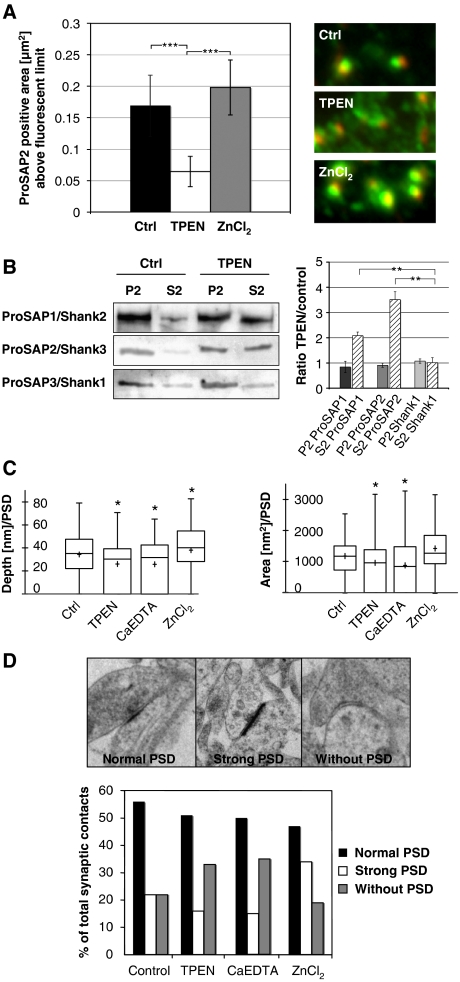
Zinc can rapidly alter the morphology of synaptic contacts and the attachment of ProSAP1/Shank2 and ProSAP2/Shank3 at PSDs. (A) A 20-min application of the zinc chelator TPEN results in the loss of defined ProSAP2/Shank3 dots (green) adjacent to presynaptic specializations as marked by Bassoon immunoreactivity (red). Measurement of the ProSAP2/Shank3-positive area above a present fluorescence limit illustrates the significant reduction within the TPEN group (control 0.17±0.055 versus 0.06±0.03) and a slight enlargement upon ZnCl2 treatment (0.2±0.05). Altogether, >500 synapses were measured for each condition and the results were corrected for inhibitory synapses, which are positive for Bassoon but a priori negative for ProSAP/Shanks. The mean number of inhibitory synapses per cell was assessed via Gepyhrin and Bassoon staining and amounted to 23.1±1.33%. (B) Western blot analysis of neuronal P2 and S2 fractions without and with short TPEN treatment shows that the zinc-binding proteins ProSAP1/Shank2 and ProSAP2/Shank3 are shifted towards the soluble fraction while the Shank1 signal stays unchanged upon TPEN application. (C) Ultrastructural analysis of PSD depth and area by electron microscopy shows a significant reduction of both parameters for TPEN and CaEDTA application and a slight enlargement in the ZnCl2 group. (D) The ultrastructural analysis of synaptic contacts according to the semiquantitative criteria ‘normal PSD', ‘strong PSD' or ‘without PSD' indicates that the percentage of synaptic contacts without a PSD is higher in the zinc chelator groups (TPEN, CaEDTA), while ZnCl2 treatment leads to the increased appearance of ‘strong' PSDs. *P=0.5; **P=0.01; ***P=0.001.