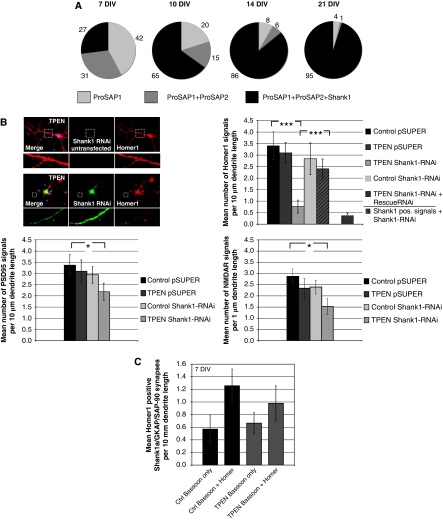
Figure 5.
Shank1 and zinc depletion significantly influence the number of synapses depending on synapse maturity. (A) ProSAP/Shank family members are recruited consecutively to postsynaptic sites. Only postsynaptic specializations containing either ProSAP1/Shank2 only, or ProSAP1/Shank2 and ProSAP2/Shank3, or all three family members are found (percentages of these PSD types are indicated at different stages of development in primary culture). During early development of synaptic contacts (DIV 7), two thirds of all PSDs do not contain any Shank1. During further development, the fraction of triply positive postsynapses steeply increases, and at DIV 21 >95% of all synapses are labelled by antibodies against all three ProSAP/Shank family members. (B) The specific downregulation of Shank1 by transfection of Shank1-RNAi significantly reduced the Homer1-positive signal after TPEN treatment. This reduction was not seen in untransfected neurons, without TPEN treatment or upon cotransfection of a Shank1-RNAi-resistant protein to rescue the loss of endogenous protein. Staining against Shank1 in Shank1-RNAi-transfected neurons indicates the overall reduction of Shank1-positive signals to about 12% in comparison to controls. Additionally, Shank1 downregulation and TPEN treatment (20 min) significantly reduced the number of PSD-95 and NMDAR-positive PSDs to about 60%. The reduction of both postsynaptic proteins was not observed when TPEN was applied to vector control transfected cells or Shank1-RNAi-transfected neurons without TPEN treatment. The cells were stained using Homer1, PSD-95 or NMDAR1 antibodies. For evaluation, fluorescent puncta along primary and secondary dendrites within the field of view were counted and the mean number of fluorescent signals was compared under the different conditions. (C) Shank1 stabilizes young synapses (7 DIV). Immunocytochemistry for endogenous Homer1 and Bassoon was performed on untreated cells and the number of Bassoon only and Bassoon and Homer1 positive signals was counted and compared with zinc-depleted cells. All cells were triply transfected with Shank1a-GFP, GKAP and PSD-95-Myc. There is no significant difference between Zn2+-depleted (Zn–) and untreated neurons. For evaluation, fluorescent puncta along dendrites within the field of view were counted and the mean number of monofluorescent (‘Bassoon only') and double-fluorescent (‘Bassoon and Homer') signals was compared. *P=0.5; **P=0.01; ***P=0.001.