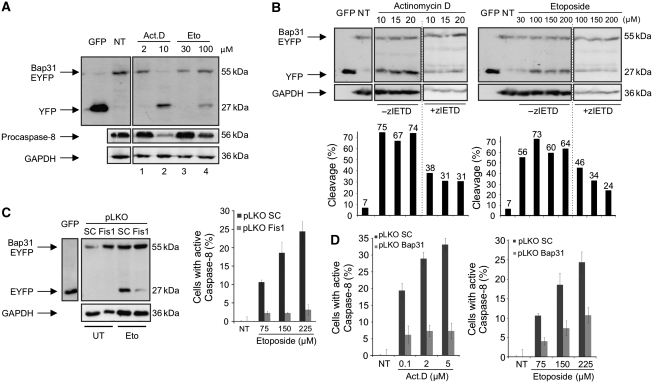
Figure 3.
Caspase-8 activation is dependent on Fis1 and Bap31 (A) Cells were transfected with the Bap31–EYFP fusion construct. Following the expression of the fusion protein, cells were treated with actinomycin D (Act.D, 2 or 10 μM) for 4 h or etoposide (Eto, 30 or 100 μM) for 30 h. Procession of Bap31–EYFP and procaspase-8 was analysed on immunoblot. Cell lysates from GFP-transfected cells were used as size indicators. NT, lysates from non-treated cells. (B) Cells were transfected with Bap31–EYFP and treated either with actinomycin D (6 h) or with etoposide (30 h) with the indicated concentrations. Cells were also cultivated in the presence or absence of the caspase-8 inhibitor zIETD-fmk during the treatment. Cell lysates from GFP-transfected cells were used as size indicators. NT, lysates from non-treated cells (top). The percentages of the cleavage of Bap31–EYFP were calculated by the intensity of the cleaved product (EYFP band) divided by the sum of intensities of the uncleaved (Bap31–EYFP band) and the cleaved (EYFP band) bands obtained from densitometry analysis using the ImageJ programme (bottom). (C) The cleavage of Bap31–EYFP was assessed in a western blot using a stable Fis1 knockdown cell line (Fis1) or a control cell line (SC) with etoposide treatment (100 μM, 30 h, left panels). Cell lysates from GFP-transfected cells were used as size indicators. The activation of caspase-8 was determined using the stable Fis1 knockdown cell line (pLKO Fis1) with etoposide treatment of the cells (30 h, right panel). pLKO SC is a control cell line for pLKO Fis1. (D) HeLa cells with stably downregulated Bap31 (pLKO Bap31) or control cells (pLKO SC) were treated with actinomycin D (23 h, left panel) or etoposide (30 h, right panel) and the activation of caspase-8 was measured.