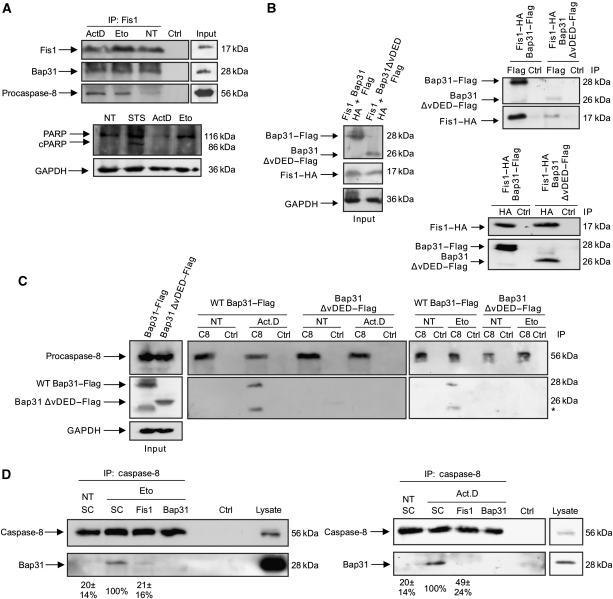
Figure 4.
Procaspase-8 is recruited to the Fis1–Bap31 platform on apoptosis induction. (A) Cells were treated with actinomycin D (2 μM) for 4 h or with etoposide (100 μM) for 28 h, and cell lysates subjected to immunoprecipitation with an antibody against endogenous Fis1. Immunoprecipitates were separated on a SDS–PAGE and subsequently probed for endogenous Fis1, Bap31, and procaspase-8 (top). The procession of poly-ADP-ribosomal polymerase (PARP) was determined in parallel using the same cell lysates before immunoprecipitation (bottom). Staurosporine (STS) was used as a positive control. cPARP, cleaved PARP. (B) Fis1–HA was co-transfected either with full-length Bap31–Flag or Bap31ΔvDED–Flag in HEK293T cells (left, input). Cell lysates were immunoprecipitated with α-Flag (right, top panels) or α-HA antibody (right bottom panels) and the immunoprecipitates were analysed for the indicated proteins by western blot using α-Flag or α-HA antibodies. (C) Full-length Bap31–Flag or Bap31ΔvDED–Flag were expressed in HEK293T cells (left, input) and cell lysates immunoprecipitated for endogenous procaspase-8 (C8) on treatment with actinomycin D (Act.D, 0.2 μM, 23 h) or with etoposide (Eto, 100 μM, 30 h). The immunoprecipitates were probed with α-caspase-8 and α-Flag antibodies (middle and right panels). NT, no treatment; * unspecific band (D) The association of caspase-8 with endogenous Bap31 was determined by co-immunoprecipitation of endogenous caspase-8 in cell lines depleted of Fis1 or Bap31 (as a control) on etoposide (Eto, 100 μM, 30 h) or actinomycin D (Act.D, 0.5 μM, 23 h) treatment. NT, no treatment. Numbers below show the percentage association between caspase-8 and Bap31 with respect to scramble-treated cells (SC), as revealed by densitometry analysis using the ImageJ programme. Figures are means±s.d. of three independent experiments.