A spicy family tree: TRPV1 and its thermoceptive and nociceptive lineage
In the current issue, Mishra and colleagues demonstrate that mice lacking somatosensory neurons in the TRPV1 lineage are completely insensitive to thermal stimuli, including both hot and cold temperatures.
EMBO J 30 3, 582–593 (2011); published online December072010
In the current issue, Mishra and colleagues demonstrate that mice lacking somatosensory neurons in the TRPV1 lineage are completely insensitive to thermal stimuli of any quality or modality, including both hot and cold temperatures. TRPV1 is a heat-gated ion channel expressed in most heat-sensitive nociceptive neurons and is the receptor for capsaicin, the pungent ingredient in ‘hot' chili peppers (Caterina et al, 1997). In the adult mouse, TRPV1 neurons are molecularly heterogeneous, but channel expression is absent in the majority of cold-sensitive neurons labelled with the menthol receptor TRPM8 and all presumptive mechanosensory neurons expressing the Mas-related G protein-coupled receptor D (Mrgprd). However, using a molecular genetic approach to label TRPV1 neurons from the inception of channel expression, the authors find that TRPV1 is a broad developmental marker for the majority of somatosensory neurons, including those expressing Mrgprd and TRPM8. Moreover, genetically targeted ablation of neurons within the TRPV1 lineage eliminates a broad range of mouse behaviours including thermosensation, thermoregulation, nociception, and pruriception, yet these animals retain normal mechanosensation. These results demonstrate that TRPV1 expression predominates embryonically, but is refined over postnatal development. Moreover, other thermosensitive and nociceptive molecules, such as TRPM8, are derived from the TRPV1 lineage even though they are not entirely co-expressed in adults. These findings expand the role of TRPV1 and show the channel has a patriarchal role as a molecular indicator of thermal and nociceptive afferent circuits.
We detect changes in our environment with specialized sensory nerve endings in the skin, many of which are activated by multiple modes of somatosensory stimuli, such as thermal and mechanical. This polymodality suggests that decoding of a specific sensory modality does not occur in the afferent, but is deciphered in higher centres such as the spinal cord or brain. However, this hypothesis has come under considerable debate with several elegant reports showing that ablation of specific afferent subtypes abrogates only a single sensory modality (Cavanaugh et al, 2009; Mishra and Hoon, 2010). For example, chemical ablation of neurons expressing TRPV1 nullifies heat pain, but not cold or mechanical sensitivity (Cavanaugh et al, 2009; Mishra and Hoon, 2010). Similarly, ablation of non-peptidergic Mrgprd neurons attenuates mechanosensation, but not thermosensation (Cavanaugh et al, 2009), suggesting that processing of heat and mechanical stimuli is separated within distinct and largely non-overlapping afferent circuits. However, ablation of neurons expressing the nociceptor-specific Na+ channel Nav1.8 attenuates mechanical pain with little effect on heat, the latter surprising as these mice are severely deficient in TRPV1 (Abrahamsen et al, 2008). These studies highlight the complexity in defining a particular neuron type and reveal the importance of cell-type specificity when considering the link between polymodality and molecular expression. Nonetheless, the presence of these ‘labelled lines' suggests that a significant component of modality decoding resides within the afferent itself.
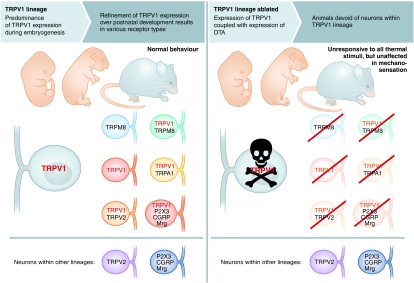
Mishra et al (2011) add to these results by generating novel transgenic mice expressing Cre recombinase via the trpv1 promoter (TRPV1-Cre), animals that were initially bred with a reporter line such that neurons within the TRPV1 lineage are genetically labelled regardless of whether TRPV1 channel expression is maintained in these cells into adulthood (Figure 1). TRPV1 transcript expression has been detected as early as embryonic day 12.5 (E12.5) in mice, with >60% of DRG neurons capsaicin sensitive by E14.5 (Hjerling-Leffler et al, 2007). However in adults, channel expression and capsaicin sensitivity is restricted to ∼30–40% of neurons, results suggesting that TRPV1 is either developmentally downregulated or that a large number of these cells undergo programmed cell death. The current study supports the former hypothesis as the genetically labelled TRPV1 lineage accounts for greater than one-half of all DRG neurons in adult mice. The intriguing question is what are these additional cell types? As expected, transgene expression co-labels with a number of TRPV1-specific markers, including the irritant receptor TRPA1, the purinergic receptor P2X3, and the neuropeptides CGRP and substance P. While neither the neurotrophic receptor TrkA nor the transcription factor Runx1 were examined, two determinants of TRPV1 expression, the transgene was limited to presumptive thermoreceptors and nociceptors as large diameter low-threshold mechanoreceptors and proprioceptors expressing TrkB and TrkC were unlabelled. However, all neurons expressing the cold-gated channel TRPM8 were, in addition to a large percentage of non-peptidergic Mrgprd neurons (Zylka et al, 2005). In the adult, TRPV1 and Mrgprd express in non-overlapping populations, whereas only ∼25% of TRPM8 neurons express TRPV1 (Zylka et al, 2005; Takashima et al, 2007). Thus, these two molecularly and functionally distinct sensory subtypes derive from the TRPV1 lineage (partially for Mrgprd), but downregulate channel expression during development by an as yet unknown mechanism(s). It is important to note that while TRPV1 marks a broad range of sensory subtypes, it does not appear to determine their specification (Caterina et al, 2000).
Figure 1.
The TRPV1 lineage predominates embryonically, yet channel expression is refined over development. However, upon embryonic ablation of neurons within this lineage, animals become unresponsive to thermal stimuli, both heat and cold, due to an apparent loss of all thermoreceptors. Nonetheless, mechanosensation remains intact, indicating that molecules and receptors involved in this modality are not within the TRPV1 lineage.
Mice lacking functional TRPV1 channels are heat-sensing impaired, but only in the high-threshold range (>50°C) (Caterina et al, 2000), and lack of deficits at moderate temperatures has been suggested to diminish TRPV1's importance in acute heat sensing. Thus, what would be the phenotype of animals lacking cells in the TRPV1 lineage? To address this question, Mishra et al crossed the TRPV1-Cre line with one in which expression of the A-subunit of diphtheria toxin (DTA) is Cre dependent, thereby generating animals (TRPV1-DTA) devoid of neurons within the TRPV1 lineage. These mice displayed no obvious abnormalities, suggesting TRPV1 expression is restricted to cells not involved in viability. However, as predicted from their labelling studies, a large percentage of DRG neurons were ablated in TRPV1-DTA mice (∼60%), including many that do not express TRPV1 in adult tissues. Predictably, expression of TRPA1 and substance P was completely eliminated, along with dramatic reductions in CGRP, P2X3, and TRPV2. However, in addition to these known markers of TRPV1 neurons, expression of TRPM8 was completely abolished, as was the majority of Mrg expression, showing again that many established TRPV1-negative neurons are a part of the TRPV1-lineage embryonically (Figure 1).
Remarkably, ablated mice were completely unresponsive to all thermal stimuli, not just noxious heat. In both hot plate and tail flick tests, these animals exhibit no behaviours within the standard assay cutoff times, even to stimuli that burned the skin. These data are consistent with that of mice in which the central terminals of TRPV1+ afferents are chemically ablated (Cavanaugh et al, 2009), further demonstrating that they are essential for heat pain. In two-temperature preference tests, TRPV1-DTA mice were unable to differentiate 30°C from 45 or 50°C, further demonstrating deficiencies in heat perception. However, it is not clear if these animals are deficient in warmth perception and it will be interesting to determine if this phenotype extends into the innocuous range using thermal preference tests at subthreshold temperatures, or with thermal gradient assays. Intriguingly, TRPV1-DTA mice are also unresponsive to the TRPM8 agonist and cold-mimetic icilin, show no preference for warmth over innocuous cool (20°C) or noxious cold temperatures (5°C), and do not react when placed on a −5°C cold plate. Thus, in addition to heat behaviours, the TRPV1-DTA line is also insensitive to cold with a phenotype even more severe than that reported for TRPM8−/− animals (Daniels and McKemy, 2007). TRPA1 expression is also abolished in these animals and might contribute to the lack of cold sensitivity, but recent analysis of mice deficient in both TRPM8 and TRPA1 suggests that TRPA1 does not have an active role in acute cold sensation (Knowlton et al, 2010). The basis for the significant differences in thermal behaviours in these animals compared TRPV1−/− and TRPM8−/− mice is not clear, but suggests that other thermotransducers act independently of these channels within the TRPV1 lineage, or that gene deletion is compensated by unknown molecular mechanisms in these cells.
In addition to the deficiencies in acute temperature detection, ablated mice have difficulty controlling core temperature. As anyone who has consumed spicy peppers can appreciate, capsaicin produces a profound hypothermic response when applied systemically; an effect that is TRPV1 dependent and, not unexpectedly, absent in ablated animals. However, when ambient temperature (25°C) shifts to either 35 or 4°C, wild-type mice become hyperthermic or hypothermic, respectively, whereas core temperature is largely unaffected in TRPV1-DTA animals. Even more intriguing is that the profound thermoregulatory changes associated with anaphylaxis and fever is also attenuated. Thus, homeostatic mechanisms controlling core temperature under a variety of conditions require the activity of neurons within the TRPV1 lineage. In addition, ablation abrogates inflammatory pain and pruriception, the former consistent with the known role of TRPV1 in inflammatory thermal hyperalgesia (Caterina et al, 2000). However, these neurons are also required for itch induced by histamine, serotonin, and PAR2 agonists, suggesting that neurons involved in pruriception are also a component of the TRPV1 lineage, further establishing a link between itch and pain.
It is important to note that mechanosensation was unaffected, indicating that the effect of ablation was not encompassing of all somatosensory stimuli. However, these results are somewhat surprising as capsaicin-sensitive neurons are polymodal in vitro, responding to mechanical stimuli in electrophysiological recordings. Moreover, as Mrgprd expression was dramatically reduced in TRPV1-DTA mice, it is striking that there were no deficits in mechanosensation considering ablation of Mrgprd+ DRG neurons attenuates responses to noxious mechanical stimuli (Cavanaugh et al, 2009). Nonetheless, Mishra et al show that neurons within the TRPV1 lineage are critical for thermosensation, nociception, and pruriception, but are dispensable in mechanosensation, adding to the mounting evidence that, at the level of the afferent nerve, the somatosensory system uses distinct modality-specific labelled lines to process sensory cues from an ever changing environment.
Footnotes
The author declares that he has no conflict of interest.
References
- Abrahamsen B, Zhao J, Asante CO, Cendan CM, Marsh S, Martinez-Barbera JP, Nassar MA, Dickenson AH, Wood JN (2008) The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 321: 702–705 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313 [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824 [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ (2009) Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci USA 106: 9075–9080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RL, McKemy DD (2007) Mice left out in the cold: commentary on the phenotype of TRPM8-nulls. Mol Pain 3: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M (2007) Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci 27: 2435–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Fisher A, Bautista DM, McKemy DD (2010) TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 150: 340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA (2010) Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Mol Cell Neurosci 43: 157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA (2011) TRPV1-lineage neurons are required for thermal sensation. EMBO J 30: 582–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y, Daniels RL, Knowlton W, Teng J, Liman ER, McKemy DD (2007) Diversity in the neural circuitry of cold sensing revealed by genetic axonal labeling of transient receptor potential melastatin 8 neurons. J Neurosci 27: 14147–14157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ (2005) Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to mrgprd. Neuron 45: 17–25 [DOI] [PubMed] [Google Scholar]