Fig. 2.
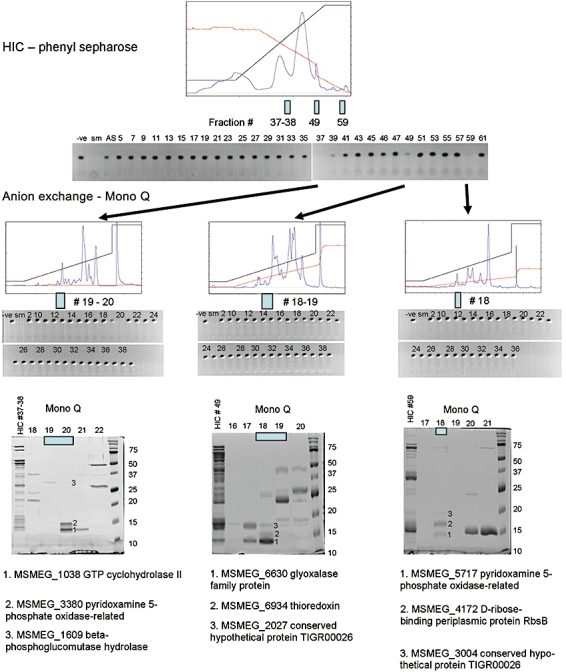
Purification protocol. Protein fractions that showed F420H2 dependent AFG1 degradation as measured by TLC were separated from M. smegmatis extracts. The ammonium sulphate precipitated proteins were first purified by hydrophobic interaction chromatography (HIC). Active fractions were further purified by anion exchange chromatography before separation by SDS-PAGE. Bands were cut from SDS-PAGE gels, digested with trypsin and analysed by LC/MS/MS. Peptides were identified from the annotated M. smegmatis genome sequence and corresponding results are shown for some of the excised bands. In the TLCs ‘-ve’ denotes no enzyme negative control and ‘sm’ denotes M. smegmatis cell extract positive control.
